Lipoma arborescens is a cause of recurrent knee joint effusions that can be treated, with good long lasting results, through arthroscopic synovectomy after thorough analysis of the magnetic resonance imaging to optimize the portals localization.
Dr. Paolo Fornaciari,
Villa St‑François, Case Postale, Chemin des Pensionnats 2‑6, CH‑1752 Villars‑sur‑Glâne, Switzerland.
E‑mail: p.fornaciari.med@gmail.com
Introduction: Lipoma arborescens (LA) is an uncommon condition that consists of a villous lipomatous proliferation of the synovial membrane. Open synovectomy has been previously selected as a curative treatment option. In recent years, some authors have published good results with arthroscopic interventions. We describe a well‑documented case of bilateral LA of the knees treated with staged arthroscopic synovectomy.
Case Presentation: A 48‑year‑old North American woman without a history of trauma presented with recurrent effusions and mild pain in both knee joints for many years. Magnetic resonance imaging examinations confirmed the diagnosis of bilateral LA with multiple villous lipomatous synovial proliferations pattern. Degenerative changes of the medial meniscus were detected bilaterally. The patient underwent bilateral arthroscopic anterior synovectomy and partial medial meniscectomy of the knee with three portal techniques. Arthroscopic the knee joint contained a large number or finger‑shaped synovial proliferations with yellowish good vascularized diffuse villous masses in the suprapatellar bursa and intercondylar fossa. The cartilage showed degenerative changes with Outerbridge Grade II to III, which was particularly severe in the femoropatellar compartment. Histopathological examination of the villous masses demonstrated papillary hypertrophy, slight hyperplasia, vascular hyperplasia with a slight degree of stromal fibrosis, and interstitial lymphoplasmacytic inflammation. The adipose cells were reduced in number in relation to a normal finding but had a normal aspect without any pathological changes. 25 months after the first operation, the patient reported pain relief with the preserved function. Magnetic resonance examination of both knee joints at the last follow‑up showed no relapse of the disease. The Knee injury and Osteoarthritis Outcome Score improved on the right knee joint from 39.3 preoperatively to 85.1 at the last follow‑up, and on the left knee joint from 54.2 preoperatively to 86.3 at the last follow‑up.
Conclusion: Arthroscopic anterior synovectomy is an efficient method of achieving good results in LA with multiple villous lipomatous synovial proliferations pattern.
Keywords: Lipoma arborescens, Villous Lipomatous Proliferation, Synovial Membrane, Knee arthroscopy.
Lipoma arborescens (LA) is an uncommon, poorly understood condition that consists of a villous lipomatous proliferation of the synovial membrane [1]. The term “arborescens” originated from the latin word arbor meaning tree, describing the macroscopic morphology of the lesion. It usually affects adults with a slight predilection for males [2]. Although typically monoarticular and found in the suprapatellar pouch of knee joints, involvement of other joints and bursae is known [3,4]. Bilateral disease is extremely rare and has been reported in 20% of cases [5]. LA usually presents with painless swelling and recurrent joint effusion. An increasing number of cases have recently been described, attributable to the increased use and diagnostic sensitivity of magnetic resonance imaging, revealing the pathognomonic findings of LA [6]. Laboratory findings and joint fluid analysis are mostly unremarkable.
The macroscopic aspect is characterized by a large number of finger‑shaped synovial proliferations, histologically composed of adipose tissue.
The etiologies most frequently advocated are developmental, traumatic, inflammatory, and neoplastic [7].
Three distinct patterns of LA have been identified; the most common of which is a diffuse villous proliferation, although a frond‑like fat synovial mass lesion and a mixed pattern have also been observed [8].
LA has been associated to many comorbidities: Rheumatoid arthritis, osteoarthritis, psoriasis, uveitis, juvenile ankylosing spondylitis [9], gout [8], trauma [9], and hypothyroidism [2].
Open synovectomy was previously selected as curative treatment option [10]. In recent years, many authors have published good results with arthroscopic interventions [11,12,13].
We describe and illustrate the clinical, histological and radiological results of a case of bilateral LA of the knees treated with staged arthroscopic synovectomy with 2‑year follow‑up.
The patient, a physically active, otherwise healthy 48‑year‑old North American woman, presented with recurrent effusions and mild pain for almost 10 years in both knee joints. By increasing her sporting activity (ski mountaineering) pain and swelling worsened. No history of knee trauma was reported. Local examination revealed a swelling of the knee joints with fullness in the suprapatellar region. Tenderness on palpation and medial meniscal tear signs were elicited in both knee joints. Flexion was slightly limited for the right knee and normal on the left knee.
The symptoms on the right knee were more pronounced. Routine laboratory tests were unremarkable, and plain radiographs of both knee joints showed moderate degenerative changes with physiological tibiofemoral axes. With the working diagnosis of bilateral gonarthritis and persistent swelling, the patient was referred for magnetic resonance investigation of both knees to select the appropriate therapy (Fig. 1a, b).
T1 and T2 weighted images showed high signal intensity of the villous projections similar to subcutaneous fat in the suprapatellar pouch. Fat‑suppressed proton density images revealed complete suppression of signal intensity. Degenerative changes of the medial meniscus were present in both knee joints. The diagnosis of LA was made. The patient underwent arthroscopic anterior synovectomy and partial medial meniscectomy of the right knee with the three portal techniques. With the use of punches and graspers, fragments of the proliferating lesion were collected from different compartments of the knee for histopathological examination. All villous projections were then resected under direct visual control using a synovial shaver 5 mm and coblation device. We first addressed the medial compartment with a craniocaudal resection from the anteromedial portal and afterward the lateral compartment in the same way using the shaver in the anterolateral portal. Following synovectomy, arthroscopic inspection confirmed complete excision of the lesion. The operation was completed with electrosurgical arthroscopic patellar denervation. 2 days postoperatively, after reabsorbing of swelling, early full weight‑bearing and functional treatment were implemented. The arthroscopic synovectomy of the contralateral knee was performed 4 months later with the same procedure including partial medial meniscectomy. The Knee injury and Osteoarthritis Outcome Score (KOOS) was collected preoperatively and at the last follow‑up.

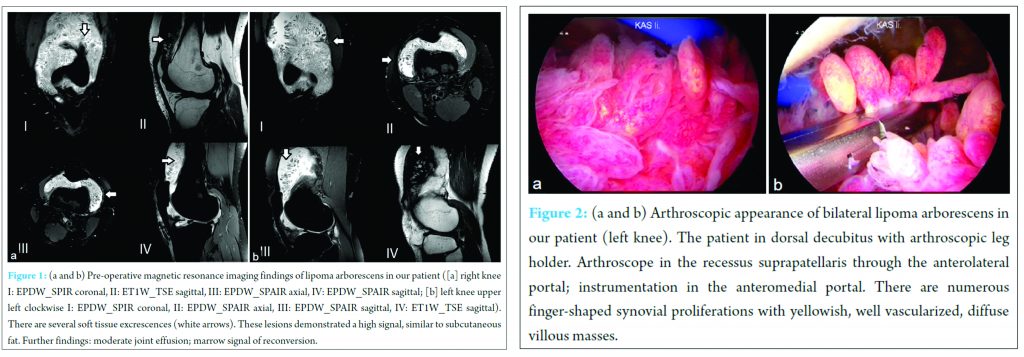
Arthroscopic the knee joint contained a large number or finger‑shaped synovial proliferations with yellowish good vascularized diffuse villous masses in the suprapatellar bursa and intercondylar fossa (Fig. 2a). The cartilage showed degenerative changes with Outerbridge Grade II to III which was particularly severe in the femoropatellar compartment. Histopathological examination of the villous masses demonstrated papillary hypertrophy, slight hyperplasia, vascular hyperplasia with a slight degree of stromal fibrosis, and interstitial lymphoplasmacytic inflammation. The adipose cells were reduced in number in relation to a normal finding but had a normal aspect without any pathological changes (Fig. 3). 25 months after the first operation, the patient reported pain relief with preserved function. Magnetic resonance examination of both knee joints at the last follow‑up showed no relapse of the disease (Fig. 4a and b). The KOOS improved on the right knee joint from 39.3 preoperatively to 85.1 at the last follow‑up, and on the left knee joint from 54.2 preoperatively to 86.3 at the last follow‑up.
Hoffa was the first author to describe the presented condition, [14]underlining the non‑neoplastic nature of the lesion. In 1988, Hallel et al. reporting on five patients, discarded the name of LA in favor of “villous lipomatous proliferation of the synovial membrane” to avoid the misleading concept of neoplasm [7].
In our histological findings, although a few residual fat cells were present, the degree of fatty change of the synovium was actually reduced relative to the normal condition, and certainly did not give the impression of fatty hypertrophy, neoplastic or otherwise.
Sola and Wright, in 1998, were the first authors reporting good results with 2‑year follow‑up after arthroscopic synovectomy in LA [11]. Due to the rarity of the condition and the few reports in the literature the best arthroscopic approach remains unknown.
About 73 articles on LA of the knee have been found after research in PubMed and references of the articles. Articles in English, German, French, Italian, and Portuguese languages have been selectioned. 34 articles describing treatment and clinical follow‑up are reported in Table 1 [14,15,16,17,18,19,20,7,21,22,23,24,25,26,11,27,2,1,28,29,30,31,32,33,10,12,34,13,35,36,5,37,38,9].
Davies and Blewitt reported a case was radiosynoviorthesis with yttrium 90 was used with relapse after 2 years [33]. Nisolle et al. described one juvenile treated with osmic acid with good results but with a limited follow‑up time of 1‑year [1]. From the analysis of this table can be sustained that the arthroscopic synovectomy gets good long lasting results with less cases of flexion deficit than open synovectomy.
The pathological villous projections are usually strictly localized in the anterior compartments not affecting the posterior joint [17], justifying a treatment with sole employment of arthroscopy with anterior portals. The massive involvement of the patellofemoral joint represents a good reason to address this compartment with additional patellar denervation.
The arthroscopic treatment, if performed thoroughly in all knee compartments, has the same efficacy as the open surgery but with less cases of post‑operative flexion deficit. Arthroscopy is less aggressive and permits early mobilization. We consider arthroscopic anterior synovectomy an efficient method of achieving lasting results in LA with diffuse villous proliferation pattern.
LA should be included in the differential diagnosis of patients with recurrent knee joint effusions. In respect of the literature, arthroscopic synovectomy gives the good long lasting results compared to other techniques. A thorough analysis of the MRI is decisive to optimize the portals localization during knee arthroscopy.
References
- 1. Nisolle JF, Boutsen Y, Legaye J, Bodart E, Parmentier JM, Esselinckx W. Monoarticular chronic synovitis in a child. Br J Rheumatol 1998;37(11):1243‑1246. [Google Scholar] [PubMed]
- 2. Kloen P, Keel SB, Chandler HP, Geiger RH, Zarins B, Rosenberg AE. Lipoma arborescens of the knee. J Bone Joint Surg Br 1998;80(2):298‑301. [Google Scholar] [PubMed]
- 3. Martinez D, Millner PA, Coral A, Newman RJ, Hardy GJ, Butt WP. Case report 745: Synovial lipoma arborescens. Skeletal Radiol 1992;21(6):393‑395. [Google Scholar] [PubMed]
- 4. Dawson JS, Dowling F, Preston BJ, Neumann L. Case report: Lipoma arborescens of the sub‑deltoid bursa. Br J Radiol 1995;68(806):197‑199. [Google Scholar] [PubMed]
- 5. Sailhan F, Hautefort P, Coulomb A, Mary P, Damsin JP. Bilateral lipoma arborescens of the knee: A case report. J Bone Joint Surg Am 2011;93(2):195‑198. [Google Scholar] [PubMed]
- 6. Feller JF, Rishi M, Hughes EC. Lipoma arborescens of the knee: MR demonstration. AJR Am J Roentgenol 1994;163(1):162‑164. [Google Scholar] [PubMed]
- 7. Hallel T, Lew S, Bansal M. Villous lipomatous proliferation of the synovial membrane (lipoma arborescens). J Bone Joint Surg Am 1988;70(2):264‑270. [Google Scholar] [PubMed]
- 8. Soler T, Rodríguez E, Bargiela A, Da Riba M. Lipoma arborescens of the knee: MR characteristics in 13 joints. J Comput Assist Tomogr 1998;22(4):605‑609. [Google Scholar] [PubMed]
- 9. Xue J, Alario AJ, Nelson SD, Wu H. Progressive bilateral lipoma arborescens of the knee complicated by juvenile spondyloarthropathy: A case report and review of the literature. Semin Arthritis Rheum 2013;43(2):259‑263. [Google Scholar] [PubMed]
- 10. Azzouz D, Tekaya R, Hamdi W, Montacer Kchir M. Lipoma arborescens of the knee. J Clin Rheumatol 2008;14(6):370‑372. [Google Scholar] [PubMed]
- 11. Sola JB, Wright RW. Arthroscopic treatment for lipoma arborescens of the knee: A case report. J Bone Joint Surg Am 1998;80(1):99‑103. [Google Scholar] [PubMed]
- 12. Yan CH, Wong JW, Yip DK. Bilateral knee lipoma arborescens: A case report. J Orthop Surg (Hong Kong) 2008;16(1):107‑110. [Google Scholar] [PubMed]
- 13. Checa A, O’Connor CR. Lipoma arborescens as an unusual cause of recurrent effusion in knee osteoarthritis: Sonographic and arthroscopic appearance. J Clin Rheumatol 2010;16(2):102‑103. [Google Scholar] [PubMed]
- 14. Hoffa A. The influence of the adipose tissue with regard to the pathology of the knee joint. JAMA 1904;43:795‑796. [Google Scholar] [PubMed]
- 15. Arzimanoglu A. Bilateral arborescent lipoma of the knee. J Bone Joint Surg Am 1957;39‑A(4):976‑979. [Google Scholar] [PubMed]
- 16. Gäde E. Ein fall von synovitis chronica villosa generalisata. Arch Orthop Unfallchir 1961;53:315‑319. [Google Scholar] [PubMed]
- 17. Weitzman G. Lipoma arborescens of the knee. Report of a case. J Bone Joint Surg Am 1965;47:1030‑1033. [Google Scholar] [PubMed]
- 18. Coventry MB, Harrison EG Jr, Martin JF. Benign synovial tumors of the knee: A diagnostic problem. J Bone Joint Surg Am 1966;48(7):1350‑1358. [Google Scholar] [PubMed]
- 19. Burgan DW. Lipoma arborescens of the knee: Another cause of filling defects on a knee arthrogram. Radiology 1971;101(3):583‑584. [Google Scholar] [PubMed]
- 20. Hermann G, Hochberg F. Lipoma arborescens: Arthrographic findings. Orthopedics 1980;3(1):19‑21. [Google Scholar] [PubMed]
- 21. Hubscher O, Costanza E, Elsner B. Chronic monoarthritis due to lipoma arborescens. J Rheumatol 1990;17(6):861‑862. [Google Scholar] [PubMed]
- 22. Edamitsu S, Mizuta H, Kubota K, Matsukawa A, Takagi K. Lipoma arborescens with hemarthrosis of the knee. A case report. Acta Orthop Scand 1993;64(5):601‑602. [Google Scholar] [PubMed]
- 23. Blais RE, LaPrade RF, Chaljub G, Adesokan A. The arthroscopic appearance of lipoma arborescens of the knee. Arthroscopy 1995;11(5):623‑627. [Google Scholar] [PubMed]
- 24. Bouraoui S, Haouet S, Mestiri H, Ennaïfar E, Chatti S, Kchir N, et al. Synovial lipoma arborescens. Ann Pathol 1996;16(2):120‑123. [Google Scholar] [PubMed]
- 25. Mestiri M, Kooli M, Charfi F, Ezzaouia K, Robbana A, Zlitni M. Lipoma arborescens of the knee: Contribution of x‑ray computed tomography. Apropos of a new case. Rev Chir Orthop Reparatrice Appar Mot 1996;82(4):340‑343. [Google Scholar] [PubMed]
- 26. Ikushima K, Ueda T, Kudawara I, Yoshikawa H. Lipoma arborescens of the knee as a possible cause of osteoarthrosis. Orthopedics 2001;24(6):603‑605. [Google Scholar] [PubMed]
- 27. Haasbeek JF, Alvillar RE. Childhood lipoma arborescens presenting as bilateral suprapatellar masses. J Rheumatol 1999;26(3):683‑686. [Google Scholar] [PubMed]
- 28. Franco M, Puch JM, Carayon MJ, Bortolotti D, Albano L, Lallemand A. Lipoma arborescens of the knee: Report of a case managed by arthroscopic synovectomy. Joint Bone Spine 2004;71(1):73‑75. [Google Scholar] [PubMed]
- 29. Erselcan T, Bulut O, Bulut S, Dogan D, Turgut B, Ozdemir S, et al. Lipoma arborescens; Successfully treated by yttrium‑90 radiosynovectomy. Ann Nucl Med 2003;17(7):593‑596. [Google Scholar] [PubMed]
- 30. Yildiz C, Deveci MS, Ozcan A, Saraçoglu HI, Erler K, Basbozkurt M. Lipoma arborescens (diffuse articular lipomatosis). J South Orthop Assoc 2003;12(3):163‑166. [Google Scholar] [PubMed]
- 31. Kim RS, Song JS, Park SW, Kim L, Park SR, Jung JH, et al. Lipoma arborescens of the knee. Arthroscopy 2004;20(8):e95‑e99. [Google Scholar] [PubMed]
- 32. Cil A, Atay OA, Aydingöz U, Tetik O, Gedikoglu G, Doral MN. Bilateral lipoma arborescens of the knee in a child: A case report. Knee Surg Sports Traumatol Arthrosc 2005;13(6):463‑467. [Google Scholar] [PubMed]
- 33. Davies AP, Blewitt N. Lipoma arborescens of the knee. Knee 2005;12(5):394‑396. [Google Scholar] [PubMed]
- 34. Santiago M, Passos AS, Medeiros AF, Sá D, Correia Silva TM, Fernandes JL. Polyarticular lipoma arborescens with inflammatory synovitis. J Clin Rheumatol 2009;15(6):306‑308. [Google Scholar] [PubMed]
- 35. Ji JH, Lee YS, Shafi M. Spontaneous recurrent hemarthrosis of the knee joint in elderly patients with osteoarthritis: An infrequent presentation of synovial lipoma arborescens. Knee Surg Sports Traumatol Arthrosc 2010;18(10):1352‑1355. [Google Scholar] [PubMed]
- 36. Utkan A, Ozkan G, Köse CC, Ciliz DS, Albayrak AL. Congenital absence of the medial meniscus associated with lipoma arborescens. Knee 2010;17(3):258‑260. [Google Scholar] [PubMed]
- 37. Xiao J, Xu Y, Wang J, Feng J, Shi Z. Bilateral knee lipoma arborescens combined with osteoarthritis in elderly patients. J Int Med Res 2011;39(4):1563‑1569. [Google Scholar] [PubMed]
- 38. Jurkiewicz A. Lipoma arborescens of the knee treated with arthroscopic synovectomy a case report and review of the literature. JBJS Case Connect 2013;2:e53. [Google Scholar] [PubMed]