This case report provides an example of the clinical findings, along with diagnostic imaging and histopathology used to identify an intratendinous calcified chondroid mesenchymal neoplasm of hamstring tendon origin.
Christopher Warburton, Medical Student at University of Miami Miller School of Medicine, Miami, Florida. E-mail: cswarburton@med.miami.edu
Introduction: We present a case report concerning calcified chondroid mesenchymal neoplasms (CCMN), a novel category of tumors that exhibit chondroid formation and contain fibronectin 1-receptor tyrosine kinase fusions.
Case Report: Our report focuses on a 73-year-old female patient who presented with pain in her right hip and gluteal region. Initially, the condition was misdiagnosed as calcific tendinitis based on X-ray imaging. However, a subsequent magnetic resonance imaging (MRI) revealed a solid lobulated mass originating from the right hamstring tendon origin, exhibiting heterogeneously hypointense T1-weighted signal, heterogeneously isointense proton density fat-suppressed signal, and heterogeneous contrast-enhancement compared to skeletal muscle. Further, investigation through computed tomography (CT) demonstrated intratumoral calcifications accompanied by erosive changes in the adjacent right ischial tuberosity. Histologic examination of a CT-guided biopsy confirmed the presence of large calcium pyrophosphate crystal deposits, along with benign chondroid tissue, thus supporting the diagnosis of CCMN. Notably, there is considerable overlap in the imaging characteristics of CCMN and the more commonly encountered calcific tendinitis (calcium hydroxyapatite depositional disease). Contrast-enhanced MRI findings play a crucial role in distinguishing between these two conditions.
Conclusion: To the best of our knowledge, this is the first documented report describing the imaging features of CCMN across multiple modalities, including radiography, CT, and MRI.
Keywords: Tumor, mass, hip.
“Calcified chondroid mesenchymal neoplasm” (CCMN) is a newly coined term used to classify a group of chondroid-forming neoplasms that harbor fibronectin 1 (FN1)-receptor tyrosine kinase fusions. These neoplasms encompass entities such as chondroblastoma-like soft-tissue chondromas and chondroid tenosynovial giant cell tumors (TGCTs) [1]. Histologically, CCMNs exhibit a lobular, multinodular architecture characterized by irregular and lace-like calcifications, as well as increased cellularity at the periphery of the nodules. The tumor cells demonstrate a stellate to polygonal shape, with moderate eosinophilic cytoplasm and nuclei eccentrically located within the chondroid matrix. Notably, certain cases of grungy calcifications exhibit features consistent with calcium pyrophosphate deposition (CPPD) [1].
In this report, we aim to describe the imaging findings of CCMNs as observed through radiography, computed tomography (CT), and magnetic resonance imaging (MRI). These imaging modalities play a crucial role in distinguishing intratendinous CCMNs from calcific tendinitis (intratendinous hydroxyapatite depositional disease [HADD]). By elucidating the distinct imaging characteristics, this study provides valuable insights into the differentiation of these two conditions.
A 73-year-old female patient was referred to our orthopedic oncology clinic for further evaluation of a “right sacral mass.” She reported a 6-month history of lower back and right gluteal pain, which radiated down her right leg and was accompanied by numbness and tingling. Despite taking non-steroidal anti-inflammatory medications and gabapentin, she experienced minimal pain relief. The patient denied experiencing fevers, changes in appetite, weight loss or gain, recent trauma, or previous surgery in the affected area. Her family history was unremarkable.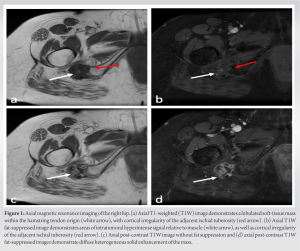
On physical examination, tenderness was noted on palpation of the right gluteal region and the upper leg around the hamstring origin of the ischial tuberosity. Hip flexion exacerbated her\symptoms and restricted the range of motion in her right hip. No apparent skin changes or gross deformities were observed. Neurovascular and motor examination did not reveal any additional weakness or sensory deficits beyond those mentioned above. The radiating pain, numbness, and tingling in the right gluteal region were likely attributed to compression of the sciatic nerve by the tumor mass in various hip positions.
X-rays taken at an external medical institution displayed soft-tissue calcification in the region of the ischial tuberosity, extending just distally into the right ischial tuberosity. This prompted further investigation through MRI. A review of the axial MRI findings (Fig. 1) revealed a well-defined, lobulated heterogeneous soft-tissue mass measuring 6.9 × 3.1 × 2.7 cm within the origin of the right hamstring tendon. The mass exhibited intermediate signal intensity on T1-weighted (T1W) sequences, resembling the signal intensity of skeletal muscle, with some areas of decreased signal intensity. Post-contrast T1W fat-saturated sequences showed intermediate signal intensity with no surrounding edema (Fig. 1d). The lesion extended toward and caused cortical irregularity of the adjacent right ischial tuberosity, which was evident on coronal MRI (Fig. 2). Post-contrast imaging demonstrated solid heterogenous enhancement of the soft-tissue mass, along with scattered areas of low signal intensity on T1W sequences, due to intralesional calcifications (Figs. 1 and 2). To further investigate the mass, a CT-guided biopsy was performed, and the scout view (Fig. 3) showed lobulated calcified mass near the right ischial tuberosity. 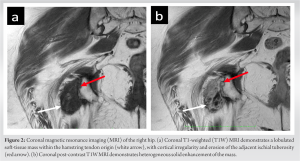 Axial CT images (Fig. 4) revealed that the calcified mass was located within the hamstring tendon origin, with cortical irregularity of the adjacent right ischial tuberosity. The presence of intratumoral calcifications was consistent with an internal chondroid matrix. Histological analysis of the biopsy (Fig. 5) revealed significant deposits of calcium pyrophosphate crystals associated with benign chondroid tissue.
Axial CT images (Fig. 4) revealed that the calcified mass was located within the hamstring tendon origin, with cortical irregularity of the adjacent right ischial tuberosity. The presence of intratumoral calcifications was consistent with an internal chondroid matrix. Histological analysis of the biopsy (Fig. 5) revealed significant deposits of calcium pyrophosphate crystals associated with benign chondroid tissue. 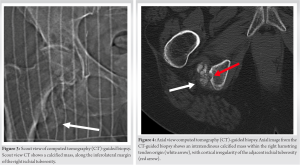 Polarized microscopy was performed and revealed small rhomboidal geometrically shaped crystals. Immunohistochemistry for the pan-TRK antibody yielded negative results, while S100 staining highlighted a few chondroid cells. These findings were indicative of a CCMN. Following histological analysis of the mass, the mass was excised surgically, ensuring that the entire tumor mass and its gross pathology were removed. Negative tumor margins were determined intraoperatively by the pathologist. Our institution’s standard is to have pathology evaluate the tumor on resection to determine negative margins before closing the patient. Postoperatively, a standard hamstring tendon tear physical therapy regimen was employed for rehabilitation. Following surgical resection, the patient proceeded with follow-up care at another institution closer to her home. However, as far as the orthopedic surgeon who excised the tumor at our institution is aware, the patient has not returned for any new or recurring symptoms.
Polarized microscopy was performed and revealed small rhomboidal geometrically shaped crystals. Immunohistochemistry for the pan-TRK antibody yielded negative results, while S100 staining highlighted a few chondroid cells. These findings were indicative of a CCMN. Following histological analysis of the mass, the mass was excised surgically, ensuring that the entire tumor mass and its gross pathology were removed. Negative tumor margins were determined intraoperatively by the pathologist. Our institution’s standard is to have pathology evaluate the tumor on resection to determine negative margins before closing the patient. Postoperatively, a standard hamstring tendon tear physical therapy regimen was employed for rehabilitation. Following surgical resection, the patient proceeded with follow-up care at another institution closer to her home. However, as far as the orthopedic surgeon who excised the tumor at our institution is aware, the patient has not returned for any new or recurring symptoms.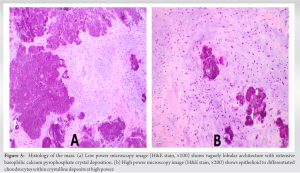
CCMN is a recently introduced term by Liu et al. in 2021 to provide a comprehensive understanding of chondroid matrix-producing neoplastic entities with FN1-receptor tyrosine kinase fusions [1]. Liu et al. observed that CCMN exhibits overlapping morphologic and genetic features of chondroblastoma-like soft-tissue chondromas and chondroid TGCTs, along with variable calcium pyrophosphate crystal deposition [1,2,3].
On one end of the spectrum, CCMN shares similarities with chondroid TGCTs. Histologically and morphologically, chondroid TGCTs display sheet-like proliferation of large epithelioid to histiocytoid mononuclear cells. Some cases exhibit hemosiderin deposition in a ring-like fashion around the cytoplasm, as well as multinucleated giant cells, which are typical of conventional TGCTs. In addition, chondroid TGCTs demonstrate the presence of geographic or nodular areas of chondroid matrix, often associated with grungy, lace-like calcifications, akin to chondroblastoma-like soft-tissue tumors, as described by Liu et al. [1]. While some chondroid TGCTs are reported to result from CSF1 fusion, others harbor the FN1 fusion [1], which prompts the consideration of reclassifying FN1 fusion subtypes of chondroid TGCTs and those exhibiting significant histological overlap with chondroblastoma-like soft-tissue tumors as CCMN.
CCMN also encompasses features of chondroblastoma-like soft-tissue tumors, which histologically manifest as cellular foci consisting of epithelioid chondrocytes of varying sizes admixed with osteoclast-like giant cells within a variable amount of chondroid matrix, frequently accompanied by lace-like calcifications [4]. However, certain FN1 fusion subtypes of chondroblastoma-like soft-tissue tumors reported by Liu et al. also exhibit TGCT features and calcium pyrophosphate deposition. The spectrum of these features is encompassed by the recently defined CCMN classification.
In the past, chondroblastoma-like soft-tissue chondromas were distinguished from TGCTs based on the absence of specific cell types, such as siderophages, foamy macrophages, and lymphocytes intermixed with mononuclear cells [4]. Chondroid TGCTs, on the other hand, feature a collagenous and hyalinized background stroma not observed in chondroblastoma-like chondromas [4]. However, Liu et al. have demonstrated that these entities are better characterized as part of a spectrum of chondroid matrix-forming tumors with translocations involving FN1 and receptor tyrosine kinase domains, including FGFR1 (without Ig domain), FGFR2, MERTK, TEK1, and NTRK11. Previously, tumors with tyrosine kinase domains containing FGFR1 (without Ig domain) and FGFR2 were categorized as a subset of soft-tissue chondromas. However, Liu et al. introduced the term CCMN to expand the classification and encompass these entities within a broader spectrum of chondroid-producing neoplasms [1,5,6].
In our case, the imaging findings of the CCMN revealed an amorphous calcified mass projecting inferiorly to the right ischial tuberosity on radiographs. MRI showed cortical irregularity of the ischial tuberosity and demonstrated a calcified low to intermediate T1W signal intensity intratendinous soft-tissue mass within the hamstring tendon origin. The mildly increased T1W signal within the tumor may be attributed to a combination of intratumoral protein content and T1 shortening due to surface interactions between microcalcifications and protons [7,8]. In addition, intralesional foci of high signal intensity were observed on proton density images, which likely corresponded to the chondroid/cartilaginous matrix of the neoplasm. Notably, there was minimal perilesional edema on fluid-sensitive sequences, which serves as a distinguishing feature from acute calcific tendinitis. Post-contrast imaging revealed a heterogeneously enhancing solid soft-tissue mass. The scattered internal foci with low signal intensity on both T1 and PD sequences were suggestive of internal mineralization within the mass. CT images (Figs. 3 and 4) further revealed mass-like amorphous calcifications within the right hamstring tendon origin, causing cortical irregularity in the adjacent ischial tuberosity.
The differential diagnosis included CCMN, HADD (calcific tendinitis), tophaceous CPPD, and calcifying aponeurotic fibroma. In this case, HADD was the primary consideration due to the presence of extra-articular, intratendinous mineralization. HADD is a common cause of intratendinous mineralization, typically affecting women between the ages of 30 and 50, primarily involving the shoulders [7,9,10,11,12,13]. The underlying pathophysiology of calcium hydroxyapatite deposition within tendons remains unclear but may be related to repeated microinjury, leading to tendon ischemia and subsequent remodeling [14,15]. CCMN and HADD share overlapping features on plain film and CT, such as intratendinous amorphous calcifications and cortical erosions. However, on MRI, HADD can exhibit a variable appearance depending on the phase of the process. In the acute or resorptive phase, HADD presents with a globular low T1W signal and a low T1-FS signal due to mineralization, accompanied by increased perilesional edema and surrounding enhancement due to inflammation [11,14]. In contrast, this case of CCMN did not demonstrate perilesional edema. Due to the similar imaging findings between HADD and CCMN, histological evaluation was essential for an accurate diagnosis. Histological studies of calcific tendinitis have revealed the presence of macrophages and multinucleated cells surrounding fragmented calcium deposits, often in proximity to capillary channels. Chondrocyte-like cells have also been reported to surround calcium deposits, which are identified as calcium hydroxyapatite crystals [15,16]. In the present case of CCMN, histological examination differed from HADD in terms of the absence of surrounding macrophages, and the calcium deposits were composed of calcium pyrophosphate crystals. However, chondrocytes were evident in both conditions.
Tophaceous CPPD, also known as tumoral pseudogout, is a rare form of CPPD that can mimic the appearance of chondroid tumors [16]. These tophaceous masses are typically periarticular and can cause erosive changes in the adjacent bones. Radiographs and CT scans demonstrate amorphous mass-like calcifications [16]. MRI findings can vary, with reported findings of low T1W signal and uniformly high or heterogeneous intermediate T2-weighted (T2W) signal [16]. Interestingly, Liu et al. also suggested the possibility that tophaceous pseudogout may be a neoplastic process involving FN1 translocations, potentially falling within the CCMN spectrum [1]. However, further research on the molecular and genetic aspects of tophaceous pseudogout is needed for confirmation.
Calcifying aponeurotic fibroma is a rare fibroblastic tumor that primarily occurs in the hands and feet of children [17,18]. While it is most commonly located within the subcutaneous layer, it has also been reported to present as an intermuscular mass [19]. Radiographically, it appears as an ill-defined soft-tissue mass with or without stippled calcifications [18,19]. CT is more sensitive in detecting calcified areas and possible bony erosions. On MRI, it typically demonstrates intermediate T1W signal intensity and heterogeneous high and low T2W signal intensity, along with intense heterogeneous contrast-enhancement [18,19].
The preferred treatment for chondroid TGCTs and soft-tissue chondromas is surgical excision [5,20]. This suggests that CCMN, which encompasses both entities, would also be amenable to surgical excision. However, as research on molecular targeted inhibitors progresses, targeted therapy of the receptor tyrosine kinases may become an alternative treatment option for CCMN [1]. These treatment approaches differ from those for intratendinous HADD, which is typically a self-limiting process managed with pain control using aspirin, non-steroidal anti-inflammatory drugs, or acetaminophen, and resolves within a few weeks [21]. Non-operative treatments, including ultrasound-guided and CT-guided barbotage, as well as steroid injections, have been shown to provide pain relief and functional recovery [22,23,24,25]. Surgical treatment is reserved for cases where minimally invasive techniques are unsuccessful.
CCMN represents a spectrum of chondroid-producing neoplasms with FN1-receptor tyrosine kinase fusion, as first described by Liu et al. in 2021. This review provides the first comprehensive evaluation of the imaging characteristics of CCMN using radiography, MRI, and CT. In our case, there was a significant overlap in the radiographic and CT imaging findings between CCMN and intratendinous HADD. Contrast-enhanced MRI plays a crucial role in the diagnosis by revealing distinctive imaging features. CCMN exhibited a mass-like appearance within the tendon, minimal perilesional edema, and diffuse heterogeneous intralesional contrast enhancement. In contrast, HADD during the acute phase displayed minimal intralesional contrast-enhancement with extensive surrounding perilesional edema. The final diagnosis of CCMN is confirmed through tissue biopsy, with surgical excision being the preferred treatment. However, further investigation into targeted therapies is imperative, as surgical excision alone may not provide a definitive cure, and recurrence is possible.
CCMN is a new classification capturing the spectrum of chondroid-producing neoplasms with FN1-receptor tyrosine kinase fusions. Although rare, in an elderly female with non-specific unilateral gluteal and lower back pain with associated soft-tissue calcifications on X-ray imaging, it is important to consider a CCMN on the differential diagnoses. To the best of our knowledge, this case is one of the first reported CCMN tumors identified after the Lie et al. classification.
When a patient presents with soft-tissue calcifications on X-ray, an MR image along with histological analysis of the calcified mass can be useful to diagnose the mass as a chondroid tumor under the recently coined CCMN tumor spectrum and provide an appropriate treatment plan. Although rare, CCMN should be included on the differential of an elderly patient presenting with non-specific localized tenderness with radiating pain, especially if X-ray imaging demonstrates a soft-tissue calcification in the associated region.
References
- 1.Liu YJ, Wang W, Yeh J, Wu Y, Mantilla JG, Fletcher CD, et al. Calcified chondroid mesenchymal neoplasms with FN1-receptor tyrosine kinase gene fusions including FGFR2, FGFR1, MERTK, NTRK1, and TEK: A molecular and clinicopathologic analysis. Mod Pathol 2021;34:1373-83. [Google Scholar | PubMed]
- 2.Chung EB, Enzinger FM. Chondroma of soft parts. Cancer 1978;41:1414-24. [Google Scholar | PubMed]
- 3.Fletcher CD, Krausz T. Cartilaginous tumours of soft tissue. Appl Pathol 1988;6:208-20. [Google Scholar | PubMed]
- 4.Cates JM, Rosenberg AE, O’Connell JX, Nielsen GP. Chondroblastoma-like chondroma of soft tissue: An underrecognized variant and its differential diagnosis. Am J Surg Pathol 2001;25:661-6. [Google Scholar | PubMed]
- 5.Shetty SK, Hegde U, Agarwal G, Sreeshyla HS. Chondroid tenosynovial giant cell tumor of temporomandibular joint. Ann Maxillofac Surg 2018;8:327-9. [Google Scholar | PubMed]
- 6.Lee JC, Jeng YM, Su SY, Wu CT, Tsai KS, Lee CH, et al. Identification of a novel FN1-FGFR1 genetic fusion as a frequent event in phosphaturic mesenchymal tumour. J Pathol 2015;235:539-45. [Google Scholar | PubMed]
- 7.Zubler C, Mengiardi B, Schmid MR, Hodler J, Jost B, Pfirrmann CW. MR arthrography in calcific tendinitis of the shoulder: Diagnostic performance and pitfalls. Eur Radiol 2007;17:1603-10. [Google Scholar | PubMed]
- 8.Henkelman RM, Watts JF, Kucharczyk W. High signal intensity in MR images of calcified brain tissue. Radiology 1991;179:199-206. [Google Scholar | PubMed]
- 9.Hayes CW, Conway WF. Calcium hydroxyapatite deposition disease. Radiographics 1990;10:1031-48. [Google Scholar | PubMed]
- 10.Bosworth BM. Calcium deposits in the shoulder and subacromial bursitis: A survey of 12,122 shoulders. J Am Med Assoc 1941;116:2477-82. [Google Scholar | PubMed]
- 11.Uhthoff HK, Loehr JW. Calcific tendinopathy of the rotator cuff: Pathogenesis, diagnosis, and management. J Am Acad Orthop Surg 1997;5:183-91. [Google Scholar | PubMed]
- 12.Faure G, Daculsi G. Calcified tendinitis: A review. Ann Rheum Dis 1983;42 Suppl 1:49-53. [Google Scholar | PubMed]
- 13.Uhthoff HK, Sarkar K. Calcifying tendinitis. Baillieres Clin Rheumatol 1989;3:567-81. [Google Scholar | PubMed]
- 14.Riley GP, Harrall RL, Constant CR, Chard MD, Cawston TE, Hazleman BL. Tendon degeneration and chronic shoulder pain: Changes in the collagen composition of the human rotator cuff tendons in rotator cuff tendinitis. Ann Rheum Dis 1994;53:359-66. [Google Scholar | PubMed]
- 15.Darrieutort-Laffite C, Blanchard, F, Le Goff B. Calcific tendonitis of the rotator cuff: From formation to resorption. Joint Bone Spine 2018;85:687-92. [Google Scholar | PubMed]
- 16.Song, K, Dong J, Zhang Y, Chen B, Wang F, Zhao J, et al. Arthroscopic management of calcific tendonitis of the medial collateral ligament. Knee 2013;20:63-5. [Google Scholar | PubMed]
- 17.Keasbey LE. Juvenile aponeurotic fibroma (calcifying fibroma); A distinctive tumor arising in the palms and soles of young children. Cancer 1953;6:338-46. [Google Scholar | PubMed]
- 18.Lee SM, Ha DH, Kang H, Rho JY. Intraarticular calcifying aponeurotic fibroma of the wrist: Mimicking gout or calcium pyrophosphate dihydrate deposition disease. Skeletal Radiol 2018;47:729-34. [Google Scholar | PubMed]
- 19.Shim SW, Kang BS, Lee CC, Suh JH, Shim HS. MRI features of calcifying aponeurotic fibroma in the upper arm: A case report and review of the literature. Skeletal Radiol 2016;45:1139-43. [Google Scholar | PubMed]
- 20.Oliva F, Venanzi R, Fratoni S, Maffulli N. Chondroma of the subcutaneous bursa of the Achilles tendon. Bull Hosp Jt Dis 2005;63:24-6. [Google Scholar | PubMed]
- 21.Speed CA, Hazleman BL. Calcific tendinitis of the shoulder. N Engl J Med 1999;340:1582-4. [Google Scholar | PubMed]
- 22.Lanza E, Banfi G, Serafini G, Lacelli F, Orlandi D, Bandirali M, et al. Ultrasound-guided percutaneous irrigation in rotator cuff calcific tendinopathy: What is the evidence? A systematic review with proposals for future reporting. Eur Radiol 2015;25:2176-83. [Google Scholar | PubMed]
- 23.Serafini G, Sconfienza LM, Lacelli F, Silvestri E, Aliprandi A, Sardanelli F. Rotator cuff calcific tendonitis: Short-term and 10-year Outcomes after two-needle us-guided percutaneous treatment--nonrandomized controlled trial. Radiology 2009;252:157-64. [Google Scholar | PubMed]
- 24.Park SM, Baek JH, Ko YB, Lee HJ, Park KJ, Ha YC. Management of acute calcific tendinitis around the hip joint. Am J Sports Med 2014;42:2659-65. [Google Scholar | PubMed]
- 25.Choudur HN, Munk PL. Image-guided corticosteroid injection of calcific tendonitis of gluteus maximus. J Clin Rheumatol 2006;12:176-8. [Google Scholar | PubMed]





