Given the acute and aggressive nature of gas gangrene, it is imperative to be vigilant with surgical debridement of necrotic tissue in addition to antibiotic treatment as soon as clinical suspicion arises.
Dr. Max Gordon, Address: Kiryat Hadassah, P.O.B: 12000, Jerusalem 91120, Israel. E-mail: max.gordon@mail.huji.ac.il
Introduction: Gas gangrene, is an aggressive and life-threatening necrotizing infection of soft tissues. We report a case of upper-limb trauma resulting in clostridial gas gangrene.
Case Report: A 36-year-old healthy male presented to our department with a left humeral shaft fracture and an open elbow fracture. During surgery wound discharge, and diffuse crepitations due to gaseous accumulation were noted around the medial elbow and forearm. Debridement and fasciotomy were performed, and IV penicillin and clindamycin were administrated. The infection spread into adjacent soft tissues, requiring repeated surgical interventions and long-term IV antibiotics till resolution.
Conclusion: Early and repeated debridements are imperative in traumatic gas gangrene, to preserve limb and function.
Keywords: gas gangrene, myonecrosis, clostridium perfringens, traumatic infection, fracture, upper limb.
Gas gangrene is a highly aggressive and sometimes lethal infection of deep soft tissues. The causative organism is clostridium species, most commonly clostridium perfringens – causing 80–90% of gas gangrene cases [1-5]. This is synonymous with myonecrosis and is characterized by rapidly progressive gangrene of the injured tissue along with the production of gas. Clostridial myonecrosis historically was a common war wound infection with an incidence of 5%, but with improvement in wound care, antisepsis, and the use of antibiotics, the incidence has fallen to 0.1% of war-related wound infections. Clostridial infections usually arise in traumatized tissue but also can arise spontaneously [1,2]. The infection involves deeper tissue such as a muscle which can lead to a rapidly spreading infection along tissue planes. The infection may develop hours to weeks after the initial trauma and inoculation [3,5]. With the best of care, including early recognition, surgical care, antibiotic treatment, and sometimes hyperbaric oxygen therapy the overall mortality rate is 20–30% and in some studies as low as 5–10%; however, if not treated the disease has a 100% fatality [5,6]. Host factors such as an immunocompromised state, diabetes mellitus, and spontaneous infections can have higher mortality rates of 67% or higher. If the infection involves the abdominal soft tissue or chest wall, the mortality rate can be as high as 60% compared to extremity infections with more favorable mortality of 5–30% [5-7].
We report a case of traumatic clostridial myonecrosis due to C. perfringens after an upper limb complicated fracture.
Our patient is a previously healthy 36-year-old man who fell during his work on a truck from a height of approximately 3 m. He was brought to the trauma room in our hospital with an isolated left upper extremity injury. He was diagnosed with a humeral shaft fracture, head of the radius fracture, and elbow dislocation, all on the left side (Fig. 1). He had a puncture wound and abrasions on his medial epicondyle area and proximal medial forearm. No other skin lesions were found. His wound was quickly washed and cleaned, and he received IV antibiotics (cefazolin 2g) under a working diagnosis of a grade I open fracture dislocation of the elbow. His elbow was reduced in the trauma room, and after reduction, placed in a posterior splint. He was admitted to the orthopedic department for definitive fixation.
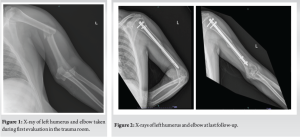
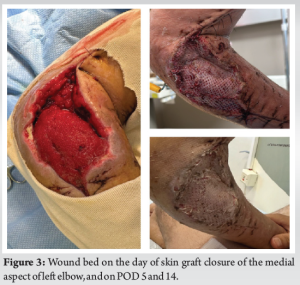
Two days after admission, the patient was taken to the operating room for definitive fixation. His humeral shaft fracture was treated with closed intramedullary nailing) MultiLoc Humeral Nailing System SYNTHES) (Fig. 2). At the time of the procedure, the operating team noted erythema and swelling of the medial aspect of the proximal forearm. The skin was incised and a tract leading into the joint was found, and a purulent discharge was drained from the wound. The wound was left open after copious irrigation of the elbow, and the purulent fluid was sent for microbial analysis. The following day, the soft tissue condition had worsened. Laboratory works at post-operative day 1 resulted in a high white blood count of 20,000 cells/ml and a C-reactive protein (CRP) value of 33.29 mg/dl (normal range 0–0.5 mg/dl). The patient was taken back to the operating room where extensive soft tissue necrosis of the subcutaneous tissue, pronator and flexor proximal muscles, and medial joint capsule were found. Radical debridement was pre-formed and the wound was left open. Cultures were still inconclusive at that point. The patient was still on intravenous cefazolin and was taken back for a third look 48 h later. Five days following the index procedure, cultures came back positive for Clostridium perfringens. After consulting our microbiologist team, IV Penicillin and Clindamycin were administered. The patient was taken back to the operating room for serial debridements circa every 48 h. On POD 17 after the index procedure, a clean, bleeding, and viable wound bed was found, and a partial thickness skin graft was performed (Fig. 3). Lab values at the end of the course were markedly improved with a CRP value of 0.99 mg/DL and normal blood count.
Since the entire medial and anterior elbow capsule were excised resulting in further destabilization of the joint, a temporary elbow transfixation (“olecranization”) of the elbow by the means of Steinman pins inserted from the olecranon the humerus and from the capitellum to the radial head was performed. The patient was discharged a few days later and completed six full weeks of IV penicillin and oral amoxicillin. Three weeks after the surgical procedure the pins were removed in the outpatient clinic. He started intense physiotherapy treatment as he suffered from marked flexor weakness of the wrist and hand as well as limited range of motion (ROM) of the elbow following the damage to his medial elbow and forearm. At last follow-up, 10 months after his discharge, his humeral fracture had healed, the elbow was stable, and ROM of the elbow was almost full with the remnant end of extension restriction alone (Fig. 4). Distal function of the wrist and hand was fully rehabilitated.

In this study, we presented a rare case of clostridial gas gangrene after trauma with isolated left upper extremity fractures and dislocation. A seemingly innocent puncture wound of the elbow was complicated by a devastating, life and limb-threatening severe gas gangrene infection leading to a long and challenging surgical course. Each procedure was associated with the dilemma of aggressively removing infected and necrotic tissue on the one hand and trying to preserve limb function as much as possible on the other hand, in the hope to avoid major disability. In our opinion the early detection of the infection and repeated, frequent trips to the operating room with simultaneous proper antibiotic treatment allowed to preserve viable tissues and eradicate the infection. A multidisciplinary team of orthopedic surgeons, microbiologists, and plastic surgeons enabled us to succeed in this mission and luckily led to a favorable outcome. Going over the literature, it is uncommon to find clostridial myonecrosis in healthy patients without risk factors, specifically diabetes mellitus. Most such cases are military injuries and involve a higher level of energy than is commonly seen in civilian injuries. In addition, the environment can be austere [8,9].
- perfringens is the culprit in most cases. This organism is found in soil and organic waste, especially if contaminated with fecal material. Healthcare workers should suspect gas gangrene if anaerobic gram-positive bacilli are present in a wound with necrosis of soft tissue and muscle. Host factors and the anatomic location of inoculation of the organisms help determine whether the bacteria will develop into a clostridial myonecrosis infection. Infection is more common in deeper penetrating wounds or wounds with crush injury and tissue ischemia [10].
The virulence of the organism depends on the exotoxins produced; Clostridium perfringens is the most pathologic with 17 known toxins, with the most toxic being the alpha-toxin, a lecithinase. These toxins allow the infection to cross over connective tissue plains, spreading into the deeper muscle tissues [11,12]. Because the infection is rapidly progressive, it is important to treat patients aggressively with antibiotics, and early surgical debridement. Clindamycin should be strongly considered because it inhibits the synthesis of clostridial exotoxins and will lessen the systemic effects of these toxins. Clindamycin is bacteriostatic and not bactericidal and should be used in conjunction with a second anti-microbial such as penicillin [13-17]. We want to acknowledge our short follow-up time, but since infection control was achieved fairly quickly with full resolution after discharge, the patient was able to achieve his functional goals at this point of time.
Gas gangrene after upper limb fractures is a rare entity. Given its acute and aggressive nature, it is imperative to be vigilant with surgical debridements of necrotic tissue in addition to antibiotic treatment. Penicillin G and clindamycin along with early surgical debridement are the need of the hour.
This is a rare case of gas gangrene that was noticed during an operation for an upper limb fracture. As it is a limb- and life-threatening infection, we emphasize the importance of aggressive debridement and an appropriate antibiotic regimen. In this case, an intramedullary nail was inserted before the final diagnosis of the infection. Nonetheless, a complete recovery was achieved.
References
- 1.Takehara M. Host defense against bacterial infection and bacterial toxin-induced impairment of innate immunity. Yakugaku Zasshi 2018;138:1249-53. [Google Scholar | PubMed]
- 2.Stevens DL, Aldape MJ, Bryant AE. Life-threatening clostridial infections. Anaerobe 2012;18:254-9. [Google Scholar | PubMed]
- 3.Hassan SA, Akhtar A, Khan M, Sheikh FN, Asghar H. “Frightening” resistant clostridial myonecrosis: A case report. Cureus 2019;11:e4539. [Google Scholar | PubMed]
- 4.Stevens DL, Bryant AE. Necrotizing soft-tissue infections. N Engl J Med 2017;377:2253-65. [Google Scholar | PubMed]
- 5.Buboltz JB, Murphy-Lavoie HM. Gas Gangrene. National Center for Biotechnology Information, U.S. National Library of Medicine. NBK537030. Treasure Island, FL: StatPearls Publishing LLC.; 2019. [Google Scholar | PubMed]
- 6.Shindo Y, Dobashi Y, Sakai T, Monma C, Miyatani H, Yoshida Y. Epidemiological and pathobiological profiles of Clostridium perfringens infections: Review of consecutive series of 33 cases over a 13-year period. Int J Clin Exp Pathol 2015;8:569-77. [Google Scholar | PubMed]
- 7.Lehnhardt M, Homann HH, Daigeler A, Hauser J, Palka P, Steinau HU. Major and lethal complications of liposuction: A review of 72 cases in Germany between 1998 and 2002. Plast Reconstr Surg 2008;121:396e-403. [Google Scholar | PubMed]
- 8.Hart GB, Lamb RC, Strauss MB. Gas gangrene. J Trauma 1983;11:991-1000. [Google Scholar | PubMed]
- 9.Prokuski L. Treatment of acute infection. J Am Acad Orthop Surg 2006;14:S101-4. [Google Scholar | PubMed]
- 10.Takazawa K, Otsuka H, Nakagawa Y, Inokuchi S. Clinical features of non-clostridial gas gangrene and risk factors for in-hospital mortality. Tokai J Exp Clin Med 2015;40:124-9. [Google Scholar | PubMed]
- 11.Srivastava I, Aldape MJ, Bryant AE, Stevens DL. Spontaneous C. septicum gas gangrene: A literature review. Anaerobe 2017;48:165-71. [Google Scholar | PubMed]
- 12.Crum-Cianflone NF. Infection and musculoskeletal conditions: Infectious myositis. Best Pract Res Clin Rheumatol 2006;20:1083-97. [Google Scholar | PubMed]
- 13.Finsterer J, Hess B. Neuromuscular and central nervous system manifestations of Clostridium perfringens infections. Infection 2007;35:396-405. [Google Scholar | PubMed]
- 14.Nichols RL, Smith JW. Anaerobes from a surgical perspective. Clin Infect Dis 1994;18 Suppl 4:S280-6. [Google Scholar | PubMed]
- 15.Shin SH, Park IK, Kang JW, Lee YS, Chung YG. Vacuum-assisted closure (VAC) using multiple foam pieces for hidden space drainage through less exposure in musculoskeletal infections. J Hand Surg Asian Pac 2018;23:369-76. [Google Scholar | PubMed]
- 16.Yang Z, Hu J, Qu Y, Sun F, Leng X, Li H, et al. Interventions for treating gas gangrene. Cochrane Database Syst Rev 2015;2015:CD010577. [Google Scholar | PubMed]
- 17.Devaney B, Frawley G, Frawley L, Pilcher DV. Necrotising soft tissue infections: The effect of hyperbaric oxygen on mortality. Anaesth Intensive Care 2015;43:685-92. [Google Scholar | PubMed]