Periprosthetic joint infection (PJI) following total hip arthroplasty (THA) sustained by Staphylococcus Caprae is extremely rare. Two-stage exchange protocol can represent a successful treatment option.
Dr. Daniele Grassa, Adult Reconstruction and Joint Replacement Service, Division of Sports Traumatology and Joint Surgery, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Largo Agostino Gemelli 8, Roma, RM 00168, Italy. Università Cattolica del Sacro Cuore, Largo Francesco Vito 1, Roma, RM, 00168, Italy. E-mail: dr.danielegrassa@gmail.com
Introduction: Total hip arthroplasty (THA) surgeries are rapidly increasing due to an aging population, leading to an increase in degenerative hip osteoarthritis. However, 1% of these patients go through prosthetic joint infection (PJI), which gives rise to implant failure with prolonged periods of patient incapacitation and higher mortality risk.
Case Report: In this article, we report an unusual case of a 62-year-old male who developed a PJI 7 months after a THA. The patient complained of groin, buttock pain, and swelling. He underwent MRI examination, which revealed the presence of a voluminous three-lobed formation with liquid content located around the prosthesis. After several attempts where microbiological samples reported negative results, a microbiological sample came out positive for Staphylococcus Caprae at the time of femoral component sonication. S. caprae is a Gram-positive bacillus belonging to the Staphylococcus spp. It is most commonly found as a commensal in goats and sheep, but it is a rare pathogen in human infections. The patient underwent two-stage revision surgery, resulting in the total resolution of the infection.
Conclusion: Staphylococcus Caprae still remains an unusual cause of infection. We report the 11th hip PJI due to S. caprae successfully treated with a two-stage exchange protocol.
Keywords: orthopedic implant-related infection, antibiotic treatment, total hip arthroplasty, periprosthetic joint infection, Staphylococcus Caprae, PJIs, two-stage revision.
Total hip arthroplasty (THA) is a safe and effective procedure that can relieve pain, increase mobility, and improve patient’s quality of life [1-3]. Prosthetic joint infection (PJI) is one of the worst complications of THA, with an incidence of around 1% following primary arthroplasty. It can occur following direct inoculation, hematogenous, or contiguous spread [4]. Such infections give rise to implant failures, which may necessitate further revision surgeries [4,5]. The most commonly detected organisms in hip PJI cases are Staphylococcus aureus and Staphylococcus epidermidis. Although infrequent, coagulase-negative Staphylococcus (CoNS) species, such as Staphylococcus Caprae, has been known to cause orthopedic infections [6]. CoNS species are typically acknowledged as a natural part of the organism, residing on the surface of healthy human skin. CoNS species are regularly found in clinical samples as contaminants and are generally not thought to possess the same pathogenic potential as the coagulase-positive S. aureus. The harmful characteristics of CoNS species are linked to their capability to generate biofilm and inhabit biomaterials [7]. Rarely, these commensal organisms can become pathogenic in humans. S. caprae has been implicated in a variety of human infections, with the highest incidence being in bone and joint infections [8]. We present a case of PJI sustained by S. caprae [9]. This report represents the eleventh case description of S. caprae as the causative microorganism for PJI, to the best of our knowledge.
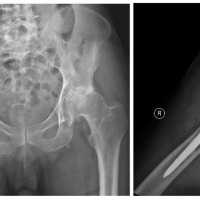
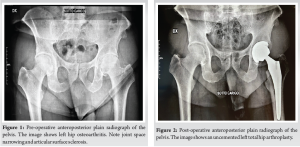
A 62-year-old male patient underwent a primary uncemented THA by direct anterior approach in June of 2020 due to the left hip end-stage osteoarthritis (Fig. 1). After 4 days, the patient was discharged home without complications (Fig. 2).
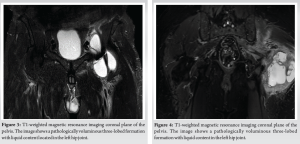
In January 2021, the patient presented with groin and buttock pain at rest and during walking, with limited range of motion (R.O.M) but with no symptoms of inflammation. MRI examination showed the presence of a voluminous three-lobed fluid-filled cyst around the implant (Fig. 3). Synovial fluid was aspirated (23 ml) and four samples were collected for cultures, all of them yielding negative results. In February 2021, the patient underwent arthroscopic synovectomy. After 1 month of follow-up, he underwent a new MRI, which showed the presence of a new voluminous cyst around the implant surrounding the nervous-vascular bundle causing paresthesia on the medial side of the thigh, and showed the same characteristics as the previous one. Subsequently, he was admitted to the hospital and a complete open resection was performed with femoral and obturator nerves neurolysis. Several samples were collected for cultures, all of them yielding negative results. The patient presented good hip function, but complained of persistent groin pain. After 7 months, he underwent 111 Indium-labeled white blood cell bone scan which documented a high leukocyte uptake around the greater trochanter. In October 2021, he underwent a new ultrasound which showed the presence of a new voluminous cyst (50 mm × 15 mm) around the implant with the same characteristics as the previous one. Synovial fluid was aspirated (20 ml) and sent for cultures. Cultures were negative. In November 2021, he was admitted for further examinations. PET-CT showed presence of increased uptake around the ileo-psoas and vastus lateralis muscles. A new ultrasound showed the presence of a voluminous cyst (42 mm × 10 mm) around the implant, and blood test revealed ESR of 42 mm, and C-reactive protein (CRP) level of 0.58 mg/dl. In January 2023, he underwent triple phase bone scan that showed increased activity isolated to the proximal femur around the femoral stem. Since there were no clear signs of infection, an isolated single stage femoral stem revision was performed in February 2023. During the surgery, several swabs were collected and sent for culture. All three samples came positive for S. caprae. As per infectious disease consultant’s recommendations, the patient was treated with 100 mg oral minocycline twice a day for 4 weeks. Two months after surgery, the patient complained of groin and buttock pain. Inflammatory markers were elevated (CRP 47.30 mg/dL), and a pelvis MRI showed a fluid containing formation with the same characteristics as the previous MRI (Fig. 4). In June 2023, he underwent a new ultrasound which showed the presence of a new voluminous cyst around the implant with the same characteristics as the previous one. Synovial fluid was aspirated (18 ml) and sent for cultures. Cultures revealed the presence of S. caprae; susceptibility to rifampin and minocycline was confirmed, which prompted the infectious disease consultant to initiate these antibiotics. The patient started taking 100 mg oral minocycline twice a day and 600 mg oral rifampin once a day. At this time, the decision was made to opt for the two-stage revision prosthesis.
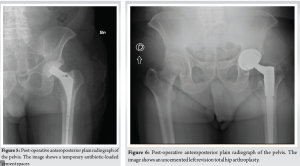
In July 2023, he underwent explant and spacer placement. During the operation, synovial and tissue samples were collected for cultures that were positive for the same pathogen. The femoral stem and acetabular component were removed, and thorough debridement and irrigation with pulse lavage were performed. Vancomycin-Gentamicin loaded bone cement was used, and a new intraoperatively molded articulating hip spacer (Spaceflex hip, G21, San Possidonio, Mo, Italy) was placed (Fig. 5). The patient was hospitalized for 10 days and treated with 100 mg IV minocycline twice a day, 600 mg IV rifampin once a day in addition 850 mg IV daptomycin once a day was given since CRP levels remained persistently elevated despite therapy with minocycline and rifampin. The day before discharge daptomycin was discontinued and a single 1500 mg IV infusion of dalbavancin was administered to achieve comprehensive coverage and an appropriate duration of therapy, considering that dalbavancin is a long-acting antibiotic. This was followed by outpatient 100 mg oral minocycline twice a day and 600 mg oral rifampin once a day. Ten days after beginning oral antibiotic therapy, minocycline was discontinued due to adverse side effects (dyspepsia and asthenia), 750 mg oral levofloxacin once a day was initiated as replacement therapy. In October 2023, the patient was readmitted for the second-stage. A spacer removal and a revision hip arthroplasty was performed using an uncemented multiholes titanium cup (Trident II Tritanium, Stryker Orthopedics, Mahwah, NJ, United States) and a modular tapered titanium revision stem (Restoration Modular Stem, Stryker Orthopaedics, Mahwah, NJ, United States) (Fig. 6). During the hospitalization, he continued the therapy with levofloxacin, rifampin, and daptomycin. At the time of the surgery, several swabs were taken and sent for culture which came out negative. The patient was discharged after 8 days without complication. Total oral antibiotic therapy with levofloxacin and rifampin was continued for 10 additional days. The last follow-up 6 months after the revision showed no sign of loosening or persistent infection.
S. caprae is a Gram-positive bacillus belonging to the Staphylococcus spp. It is a common commensal in goats and sheep, but it is a rare pathogen in human infections [10]. There have been reports of PJIs, although S. caprae still remains an unusual cause of infection [11]. Despite being acknowledged as a commensal organism in animals, only 20% of individuals who contract S. caprae infections have any documented history of animal interaction. In our specific case, there was no reported recent contact with animals or engagement in high-risk activities. While orthopedic hardware infections have a polymicrobial cause due to contamination from the traumatic and dirty wound, 60% of PJIs tend to be monomicrobial [11]. Due to its extremely low incidence, there is no unanimous agreement on the optimal therapeutic approach for S. caprae PJIs. Treatment options for PJIs include debridement, antibiotics, and implant retention (DAIR), two-stage revision exchange, one-stage revision exchange, major partial one-stage revision (removing either the femoral stem or acetabular shell), and minor partial one-stage revisions (where only the femoral head and/or acetabular liner are replaced). Although there have been previous authors reporting successful treatment of S. caprae PJIs with these treatment options [8-12], the best method has not been established. In our specific case, due to the negative results obtained from the initial wound samples, we proceeded with partial one-stage revision, removing only the femoral stem due to the suspicion of aseptic loosening of the femoral stem. The femoral stem was sent for sonication. Seven days later, the presence of S. caprae was detected. We proceeded with a two-stage revision since according to literature that it is the protocol which allows the lowest rates of reinfection [13]. Two-stage revision THA often involves severe bone loss that presents the treating surgeon with a complex reconstructive challenge with an increased risk of dislocation [14-17]. About the antibiotic treatment administered, we acknowledge that there is no consensus on the best antibiotic therapeutic approach for S. caprae. The existing medical literature supports the use of combination of fluoroquinolone with rifampicin [11]. In our specific case, we used a combination of minocycline, rifampin, daptomycin, and dalbavancin. Rifampin inhibits bacterial DNA-dependent RNA polymerase and had the advantage of good oral bioavailability, allowing us to continue adequate long-term outpatient therapy [18]. Successful treatment was accomplished with a two-stage exchange protocol and a targeted antibiotic therapy with good function outcomes, the left hip was pain-free without any clinical signs of infection and with a full R.O.M.
S. caprae represents an extremely rare cause of hip PJI. There is paucity of data on the best surgical and antibiotic management in literature. We report the 11th case successfully treated with a two-stage exchange protocol.
- caprae represents an extremely rare cause of hip PJI. Due to its extremely low incidence, there is no unanimous agreement on the optimal surgical and antibiotic management for S. caprae PJIs. The patient was treated with the two-stage protocol.
References
- 1.Learmonth ID, Young C, Rorabeck C. The operation of the century: Total hip replacement. Lancet 2007;370:1508-19. [Google Scholar | PubMed]
- 2.De Martino I, De Santis V, D’Apolito R, Sculco PK, Cross MB, Gasparini G. The Synergy cementless femoral stem in primary total hip arthroplasty at a minimum follow-up of 15 years. Bone Joint J 2017;99-B:29-36. [Google Scholar | PubMed]
- 3.De Martino I, De Santis V, Sculco PK, D’Apolito R, Poultsides LA, Gasparini G. Long-Term clinical and radiographic outcomes of porous tantalum monoblock acetabular component in primary hip arthroplasty: A minimum of 15-year follow-up. J Arthroplasty 2016;31:110-4. [Google Scholar | PubMed]
- 4.Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: The incidence, timing, and predisposing factors. Clin Orthop Relat Res 2008;466:1710-5. [Google Scholar | PubMed]
- 5.Clohisy JC, Calvert G, Tull F, McDonald D, Maloney WJ. Reasons for revision hip surgery: A retrospective review. Clin Orthop Relat Res 2004;429:188-92. [Google Scholar | PubMed]
- 6.Toms AD, Davidson D, Masri BA, Duncan CP. The management of peri-prosthetic infection in total joint arthroplasty. J Bone Joint Surg Br 2006;88:149-55. [Google Scholar | PubMed]
- 7.Becker K, Heilmann C, Peters G. Coagulase-negative staphylococci. Clin Microbiol Rev 2014;27:870-926. [Google Scholar | PubMed]
- 8.Gowda A, Pensiero AL, Packer CD. Staphylococcus caprae: A skin commensal with pathogenic potential. Cureus 2018;10:e3485. [Google Scholar | PubMed]
- 9.Domashenko P, Foukarakis G, Kenanidis E, Tsiridis E. A rare case of Staphylococcus caprae-caused periprosthetic joint infection following total hip arthroplasty: A literature review and antibiotic treatment algorithm suggestion. Cureus 2023;15:e39471. [Google Scholar | PubMed]
- 10.Vandenesch F, Eykyn SJ, Bes M, Meugnier H, Fleurette J, Etienne J. Identification and ribotypes of Staphylococcus caprae isolates isolated as human pathogens and from goat milk. J Clin Microbiol 1995;33:888-92. [Google Scholar | PubMed]
- 11.Seng P, Barbe M, Pinelli PO, Gouriet F, Drancourt M, Minebois A, et al. Staphylococcus caprae bone and joint infections: A re-emerging infection? Clin Microbiol Infect 2014;20:1052-8. [Google Scholar | PubMed]
- 12.Couto I, Pereira S, Miragaia M, Sanches IS, De Lencastre H. Identification of clinical staphylococcal isolates from humans by internal transcribed spacer PCR. J Clin Microbiol 2001;39:3099-103. [Google Scholar | PubMed]
- 13.Engesæter LB, Dale H, Schrama JC, Hallan G, Lie SA. Surgical procedures in the treatment of 784 infected THAs reported to the Norwegian Arthroplasty Register. Acta Orthop 2011;82:530-7. [Google Scholar | PubMed]
- 14.Mancino F, Cacciola G, Di Matteo V, De Marco D, Greenberg A, Perisano C, et al. Reconstruction options and outcomes for acetabular bone loss in revision hip arthroplasty. Orthop Rev (Pavia) 2020;12:8655. [Google Scholar | PubMed]
- 15.Waddell BS, De Martino I, Sculco T, Sculco P. Total hip arthroplasty dislocations are more complex than they appear: A case report of intraprosthetic dislocation of an anatomic dual-mobility implant after closed reduction. Ochsner J 2016;16:185-90. [Google Scholar | PubMed]
- 16.De Martino I, D’Apolito R, Nocon AA, Sculco TP, Sculco PK, Bostrom MP. Proximal femoral replacement in non-oncologic patients undergoing revision total hip arthroplasty. Int Orthop 2019;43:2227-33. [Google Scholar | PubMed]
- 17.Mancino F, Jones CW, Sculco TP, Sculco PK, Maccauro G, De Martino I. Survivorship and clinical outcomes of constrained acetabular liners in primary and revision total hip arthroplasty: A systematic review. J Arthroplasty 2021;36:3028-41. [Google Scholar | PubMed]
- 18.Achermann Y, Eigenmann K, Ledergerber B, Derksen L, Rafeiner P, Clauss M, et al. Factors associated with rifampin resistance in staphylococcal periprosthetic joint infections (PJI): A matched case-control study. Infection 2013;41:431-7. [Google Scholar | PubMed]