Tumoral calcinosis is a rare condition that should be considered in the differential diagnosis for periarticular calcifications, particularly in younger patients. The potential for intra-articular extension is a distinctive feature of tumoral calcinosis Surgical excision is the primary treatment modality. Due to the risk of recurrence, careful monitoring post-surgery is necessary to address any regrowth or related issues.
Dr. Manoj Kumar, Assistant Professor, Department of Orthopaedic Surgery, Maulana Azad Medical College, Bahadur Shah Marg, New Delhi - 110 02, India. E-mail: dr.mnjlohia@gmail.com
Introduction: Tumoral calcinosis is a rare hereditary condition characterized by the deposition of calcium phosphate and hydroxyapatite in periarticular soft tissues. First described by Giard and Duret in 1898 and later detailed by Inclan in 1943, this condition has often been confused with other forms of periarticular calcification. Tumoral calcinosis predominantly affects young males and is typically found around major joints, such as the shoulder, elbow, hip, ankle, and wrist.
Case Report: We present a unique case of tumoral calcinosis in a 12-year-old boy, exhibiting intra-articular extension within the knee – an unprecedented finding in the literature. The patient had a 2-year history of progressive, nodular swelling around the left knee, initially presenting as painless nodules that later became painful and ulcerated. Radiographic and CT imaging revealed a massive, lobulated calcific mass extending from the superior patella to the pre-patellar region, including an intra-articular component.
Diagnosis and Management: The diagnosis was confirmed through imaging and histopathological examination following surgical excision. Histology showed calcified masses surrounded by giant cells, epithelioid cells, and fibrosis. Treatment involved complete surgical excision of the calcific masses, followed by lavage of the knee joint. The patient showed no recurrence at a 3-year follow-up.
Discussion: Tumoral calcinosis is often misdiagnosed due to its similarity to other conditions involving soft-tissue calcification. This case underscores the importance of differentiating tumoral calcinosis from other disorders like calcinosis cutis and soft-tissue sarcomas. Genetic studies have identified mutations in GALNT3 and FGF23 genes, which contribute to the disorder’s pathogenesis by disrupting phosphate metabolism. Effective management requires a combination of surgical excision and phosphate regulation.
Conclusion: Tumoral calcinosis, though rare, should be considered in differential diagnoses for periarticular calcifications, especially in younger patients. This case highlights the need for a multi-modal diagnostic approach and emphasizes that tumoral calcinosis can present with intra-articular involvement, a feature not previously reported in the knee joint. Long-term follow-up is essential due to the potential for recurrence.
Keywords: Tumoral calcinosis, periarticular calcification, intra-articular calcification, genetic mutations, surgical management.
Tumoral calcinosis is a very rare clinical entity encountered in clinical practice. It is usually a hereditary disorder characterized by single or multiple painless periarticular masses deposition without any functional disability. The clinical entity finds it first mention in medical literature by Giard and Duret 1898 and 1899 [1,2]. It was furthered studied by Teutschlaender from 1930 to 1950 at which time it came to known as Teutschlaender disease as per European literature [3,4]. In 1943, Inclan described the entity in American literature which became a pivotal article for the diagnosis and management of tumoral calcinosis. Inclan et al. differentiated the calcification from dystrophic and metastatic calcification specifically from calcifications from real dystrophy, connective tissue disease, and hormonal imbalance [5]. Tumoral calcinosis is usually characterized by deposition of calcium phosphate and hydroxyapatite (Ca10(PO4)6 (OH)2) salts in different peri-articular soft-tissue regions [6]. The term tumoral calcinosis has been widely misused to describe any massive periarticular calcification. However, it refers to hereditary condition with massive periarticular calcifications. The radiologist plays a critical role in avoiding unnecessary invasive procedures and in guiding the selection of appropriate tests that can result in a conclusive diagnosis of tumoral calcinosis [7]. Tumoral calcinosis usually found to have sex predilection for males and usually found in the second decade of life. Tumoral calcinosis is usually found around the large joints of bodies such as shoulder, elbow, hip, ankle, and wrist. There are also reported cases of such calcifications around spine, temporomandibular joint, popliteal space, meta carpals, and meta tarsals [8]. There was only one reported case of such disorder around the knee described as pre-patellar tumoral calcinosis [6]. There is no reported case of tumoral calcinosis around the knee with intra-articular extension. The index case study elaborated below is the first of its kind with intra-articular extension in a 12-year-old boy. They usually present as lobulated periarticular calcific masses mimicking soft-tissue sarcoma. Variants are described for the above clinical entity such as primary and secondary. Primary is associated with normal serum calcium levels and hyperphosphatemia. Histologically, it consists of calcium granules surrounded by epitheloid cells and multi-nucleated giant cells [9]. Recent studies revealed autosomal recessive mutations, specifically to genes GALNT3 (GaINAc-transferase3) and FGF23 (fibroblast growth factor-23), which induce metabolic dysregulation of phosphate suggesting a post-translational defect [10,11].
A 12-year-old boy presented to us with chronic nodular swelling in the left knee for the last 2 years. Two years back the boy noticed a single nodular swelling on the anterior aspect of the knee which gradually progressed to multiple swellings on the anterior aspect of knee. Swelling was associated with mild intensity, dull aching, and non-radiating pain over the anterior aspect of knee which progressed to moderate intensity over period of time. Skin was intact initially and when the boy presented to us there was a hard nodular mass over the ulcerated skin. However, there was no restriction of range of motion or any hindrance in activities of daily living. Patient had no history of repetitive trauma or metabolic disorders in the family as proposed in the previous theories of tumoral calcinosis. He also does not have any history of any other swellings in the body or familial history of tumors. He has no associated comorbidities. On examination, about seven swellings were found on the anterior aspect of the left knee each of about 2 × 3 cm size, hard consistency, skin over the medial and inferior swellings was normal but the skin over the lateral swellings was erythematous and ulcerated. Swelling was not fixed to any underlying tissues. Swelling was does not have any particular extension as the swelling did not move with ROM of knee. ROM was however normal. However patellar crepitus was found on examination. No evidence of any other swelling in the body. Distal neurovascular examination was within normal limits (Fig. 1 and 2).
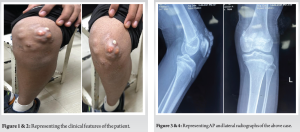
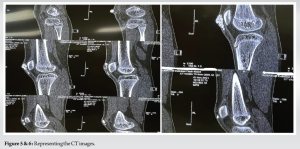
AP and lateral radiographs show cobblestone appearing calcific mass extending from superior aspect of patella extending to the inferior aspect along the pre-patellar region. Intra-articular calcific mass was also found on the radiograph with connection the major mass. Computer Tomography scan was done which confirmed the tumoral calcinosis along the pre-patellar region and a separate intra-articular calcific mass. Complete blood count was found within normal limits, serum calcium, phosphate, alkaline phosphatase, uric acid, and Vitamin D levels were found to be in the normal range when metabolic profile was done (Fig. 3-6).
Management
The patient was managed by open excision and biopsy. Intraoperatively calcific nodular masses were found in the pre-patellar region which was excised. Medial parapatellar approach was used to see intra-articular masses. Caseous material with chalky white hard multiple crystals was found in the knee. Removal of the debris was done along with lavage with normal saline to remove remnants. Histopathological examination revealed several calcified masses with surrounding fibrosis and encapsulation under low power. High power magnification revealed calcified mass in the center surrounded by giant cells, epitheloid cells, and inflammatory reaction. No recurrence was found in the follow-up over 3 years.
Tumoral calcinosis is a rare clinical entity found in clinical practice and often referred to as Teutschlaender disease. Unfortunately, the term tumoral calcinosis has been liberally and imprecisely used to describe any massive collection of periarticular calcification, although this term actually refers to a hereditary condition associated with massive periarticular calcification. The inconsistent use of this term has created confusion throughout the literature [7]. It is a histopathological entity often resembling neoplasm and the calcifications are composed of calcium hydroxyapatite and calcium phosphate. The index case study also resembled a neoplasm and hence the differentials like metastatic calcification secondary to tumor and calcinosis cutis found in immunological disorders were considered. However intra-articular extension and mass extending from superior pole of patella to the inferior aspect posed a challenge in considering the diagnosis. Tumoral calcinosis is usually found in the first and second decade of life suggesting a strong hereditary association of the disease. It usually presents in young adults and adolescents as painless, large tumor-like masses surrounding the large joints which may lead to limitation in range of motion. Pain is not usually a feature unless it is compressing a local nerve. Spontaneous regression of the tumoral calcinosis is found in pediatric population [12]. However, there is documentation of tumoral calcinosis in elderly [13]. The joints commonly affected include the elbow, hip, shoulder, ankle, and wrist. Furthermore, there are reported cases occurring in the spine, temporomandibular joint, metacarpal, metatarsal, and popliteal space [14]. There was only a single case of tumoral calcinosis reported around the knee in a 59-year-old previously in the patellar region [6]. However, the index case described above is the first case where tumoral calcinosis is found in the pre-patellar region with intra-articular region. The above case also presented with chalky white discharge and ulceration as expected with tumoral calcinosis. The location of swelling is along the extensor surface of the joint along the anatomic distribution of bursa. Further metabolic studies of disease revealed in patients with tumoral calcinosis revealed fractional decrease in phosphate excretion, increased Vitamin D formation, and a normal dynamic response to parathyroid hormone in proximal convoluted tubules [15-17]. Although the pattern of inheritance is still debated, the generally accepted mode of transmission is autosomal dominant with variable expressivity. However, recent discoveries in the field of genetics have identified autosomal recessive mutations, specifically to genes GALNT3 and FGF23, which induce metabolic dysregulation of phosphate, suggesting a post-translational defect [10,11]. Various other mechanisms have been described to understand the pathogenesis of the disorder such as (a) repetitive trauma leading to reparative dysfunction, (b) periarticular forces dissecting histiocytic aggregates that initiate osteoclastic activity, and (c) hemorrhage from microtrauma causing an exaggerated reparative response [18]. Despite these scientific advancements in understanding tumoral calcinosis, this catchy term became increasingly prevalent in the clinical literature. The term became incorporated in texts as primary tumoral calcinosis (also known as idiopathic tumoral calcinosis or familial tumoral calcinosis), which referred to the original disease reported by Inclan et al., and as secondary tumoral calcinosis, which referred to calcified masses associated with an identifiable condition. Laboratory results of tumoral calcinosis include (a) Hyperphosphatemia (b) less frequently normophosphatemia (c) Normocalcemia (d) Normal or elevated serum 1,25-dihydroxy–Vitamin D level (e) Normal parathyroid hormone level (f) Normal glomerular filtration (g) Negative results for antinuclear, anti-Smith, anticentromere, and anti-scleroderma antibodies.
Radiographic features
Tumoral calcinosis has a typical appearance on radiographs: amorphous, cystic, and multilobulated calcification located in a periarticular distribution. Axial CT better delineates the calcific mass. The cystic appearance shows fluid-fluid levels caused by calcium layering and commonly termed the sedimentation sign [19]. However, the lesion may appear homogeneous, suggesting a reduced metabolic activity and a lower likelihood of growth. Erosion or osseous destruction by adjacent soft-tissue masses is absent, another distinguishing finding of tumoral calcinosis. MR imaging with T2-weighted sequences generally shows inhomogeneous high signal intensity even though there is a large amount of calcification [20]. Histopathologic features of tumoral calcinosis. (a) High-power light microscopy shows a calcified mass in the center of the field with foreign body inflammatory reaction, giant cells, and surrounding fibrosis. (b) Low-power light microscopy shows several calcified masses with chronic inflammatory and fibrotic encapsulation. Tumoral calcinosis usually represents soft-tissue neoplasm as is with the index case described above. High index of suspicion is needed along with consideration of alternate diagnosis should be done. The disorders mimicking tumoral calcinosis are calcinosis of chronic renal failure, calcinosis universalis, calcinosis circumscripta, calcific tendonitis, synovial osteochondromatosis, synovial sarcoma, osteosarcoma, myositis ossificans, tophaceous gout, and calcific myonecrosis. Surgical excision is the treatment modality of choice for tumoral calcinosis. Recurrences are common due to poor circumscription, especially when it is actively progressing. Phosphate depletion in both normo- and hyperphosphatemia has proved to have variable success. Resistant cases are thought to be related to a late-stage tumoral calcinosis lesion surrounded by an impeding fibrous layer that prevents ion exchange. However, surgical excision combined with phosphate deprivation (using aluminum hydroxide) in conjunction with acetazolamide synergistically lowers hyperphosphatemia and has proved to be the most effective therapy. Other therapies, including systemic steroid therapy and radiation therapy, have not been proved to be effective [21,22].
Tumoral calcinosis is a rare clinical entity and its diagnosis needs a high degree of suspicion. It must be considered as a differential in soft-tissue calcification around large joints of the body. Diagnosis requires a multi-modality approach including clinical, radiological, biochemical, and histopathological investigations. It occurs due to a hereditary defect in phosphate excretion. Complete surgical excision with phosphate deprivation is the treatment of choice. Long-term follow-ups are required as recurrences are reported.
Tumoral calcinosis, though rare, should be included in the differential diagnosis for periarticular calcifications, especially in younger patients. The unique presentation of intra-articular extension in this case underscores the importance of a multi-modal diagnostic approach involving clinical, radiological, biochemical, and histopathological assessments. Effective management relies on surgical excision and careful monitoring, given the risk of recurrence. Awareness of the genetic underpinnings of the disorder can also guide clinicians in understanding its pathogenesis and informing families about potential implications.
References
- 1.Giard A. Sur la calcification hibernale. C R Soc Biol 1898;10:1013-5. [Google Scholar | PubMed]
- 2.Duret MH. Tumours multiples et singulieres des bourses sereuses (endotheliomes, peutetre d’origine parasitaire). Bull Mem Soc Anat Paris 1899;74:725-33. [Google Scholar | PubMed]
- 3.Teutschlaender O. Die lipoido-calcinosis oder lipoidkalkgicht. Beitr Pathol Ana 1949;110:402-32. [Google Scholar | PubMed]
- 4.Teutschlaender O. Aur kenntnis der lipoido-calcinosis progrediens. Zentralbl Allg Pathol 1951;87:1-15. [Google Scholar | PubMed]
- 5.Inclan A, Leon P, Camejo MG. Tumoral calcinosis. J Am Med Assoc 1943;121:490-5. [Google Scholar | PubMed]
- 6.Dim EM, Ubaha AG, Usendiah IB, Dim UM, Oforjigha-Dim CW, Essien U. Prepatellar tumoral calcinosis mimicking soft tissue sarcoma: A case report and review of literature. IBOM Med J 2023;16:347-50. [Google Scholar | PubMed]
- 7.Olsen KM, Chew FS. Tumoral calcinosis: Pearls, polemics, and alternative possibilities. Radiographics 2006;26:871-85. [Google Scholar | PubMed]
- 8.Fathi I, Sakr M. Review of tumoral calcinosis: A rare clinico-pathological entity. World J Clin Cases 2014;2:409. [Google Scholar | PubMed]
- 9.Chew FS, Crenshaw WB. Idiopathic tumoral calcinosis. AJR Am J Roentgenol 1992;158:330. [Google Scholar | PubMed]
- 10.Topaz O, Shurman DL, Bergman R, Indelman M, Ratajczak P, Mizrachi M, et al. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet 2004;36:579-81. [Google Scholar | PubMed]
- 11.Benet-Pages A, Orlik P, Strom TM, Lorenz-Depiereux B. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum Mol Genet 2005;14:385-90. [Google Scholar | PubMed]
- 12.Niali DM, Fogarty EE, Dowling FE, Moore DP. Spontaneous regression of tumoral calcinosis in an infant; a case report. J Paediatr Surg 1998;33:1429-31. [Google Scholar | PubMed]
- 13.Croock AD, Silver RM. Tumoral calcinosis presenting as adhesive capsulitis: Case report and literature review. J Am Coll Rheumatol 1987;30:455-8. [Google Scholar | PubMed]
- 14.Hammert WC, Lindsay LR. Tumoral calcinosisor is it? A case report and review. Hand (N Y) 2009;2013:119-22. [Google Scholar | PubMed]
- 15.Mitnick PD, Goldfarb S, Statopolsky E, Lemann J Jr., Gray RW, Agus ZS. Calcium and phosphate metabolism in tumoral calcinosis. Ann Intern Med 1980;92:482-7. [Google Scholar | PubMed]
- 16.Prince MJ, Schaefer PC, Goldsmith RS, Chausmer AB. Hyperphosphatemic tumoral calcinosis. Ann Intern Med 1982;96:586-91. [Google Scholar | PubMed]
- 17.Zerwekh JE, Sanders LA, Townsend J, Pak CY. Tumoral calcinosis: Evidence for concurrent defects in renal tubular phosphorus transport and in 1 alpha,25-dihydroxycholecalciferol synthesis. Calcif Tissue Int 1980;32:1-6. [Google Scholar | PubMed]
- 18.Slavin RE, Wen J, Kumar D, Evans EB. Familial tumoral calcinosis: A clinical, histopathologic, and ultrastructural study with an analysis of its calcifying process and pathogenesis. Am J Surg Pathol 1993;17:788-802. [Google Scholar | PubMed]
- 19.Smack D, Norton SA, Fitzpatrick SE. Proposal for pathogenesis-based classification of tumoral calcinosis. Int J Dermatol 1996;35:265-71. [Google Scholar | PubMed]
- 20.Martinez S, Vogler JB, Harrelson JM, Lyles KW. Imaging of tumoral calcinosis: New observations. Radiology 1990;174:215-22. [Google Scholar | PubMed]
- 21.Yamaguchi T, Sugimoto T, Imai Y, Fukase M, Fujita T, Chihara K. Successful treatment of hyperphosphatemic tumoral calcinosis with longterm acetazolamide. Bone 1995;16:247S-50. [Google Scholar | PubMed]
- 22.Lufkin EG, Wilson DM, Smith LH, Bill NJ, DeLuca HF, Dousa TF, et al. Phosphorus excretion in tumoral calcinosis: Response to parathyroid hormone and acetazolamide. J Clin Endocrinol Metab 1980;50:648-53. [Google Scholar | PubMed]