Visual case report stressing clinical suspicion of necrotizing fasciitis and shared decision-making
Dr. Zachary Jodoin, UT Health San Antonio, Department of Orthopaedics, San Antonio, TX 78229 Email: jodoin@uthscsa.edu
Introduction: It is well known that diabetic patients have impaired wound healing, increased susceptibility to infection, and harbor tissue that supports the growth of gas-producing infections. Necrotizing fasciitis (NF) is an uncommon soft-tissue infection characterized by extensive necrosis of subcutaneous tissue and fascia with relative sparing of the skin and muscle tissues. The majority of gas-producing infections are polymicrobial in nature, and therefore, NF with Staphylococcus aureus as a single etiologic agent is exceedingly uncommon.
Case Report: This is a case of a 46-year-old male that developed gas-forming NF and abscesses from methicillin-sensitive S. aureus (MSSA) after a complicated course involving undiagnosed type 2 diabetes mellitus (T2DM), diabetic ketoacidosis, and bacteremia. The disease course presented relatively slowly with mild systemic symptoms, knee pain, erythema, and edema, but steadily progressed over days leading to an elevated level of care. Multidisciplinary care was necessary to treat the patient, including surgical and intravenous antibiotic therapies. The patient’s care was prolonged due to decreased patient compliance with recommended therapies and difficulty with appropriate shared decision-making.
Conclusion: Although NF caused by monomicrobial infection with methicillin-resistant S. aureus has been previously reported, awareness of this condition remains limited, especially with concomitant gas formation. Physicians should have a high index of suspicion for NF with MSSA as a potential etiologic agent when treating patients with symptoms of a necrotizing soft-tissue infection, particularly those with underlying T2DM or a history of recent needle puncture. By engaging in shared decision making, health outcomes in these serious infections can be optimized.
Keywords: Necrotizing soft-tissue infection, shared decision-making, infectious disease, wound care, wound vacuum-assisted closure therapy.
Diabetic patients have impaired wound healing, increased susceptibility to infection, and associated comorbidities [1-3]. The spectrum of soft tissue infections in diabetic patients consequently ranges widely from indolent to life-threatening. Notably, diabetes confers an increased risk of developing gas-producing infections [4]. Necrotizing fasciitis (NF) is an uncommon soft-tissue infection characterized by extensive necrosis of subcutaneous tissue and fascia with relative sparing of the muscle and skin [5]. NF was recently subdivided into three types: Type I, mixed infection, type II, Group-A β-hemolytic Streptococcus (GABHS) with or without Staphylococcus aureus, and type III, marine vibrio. Type I constitutes 55–90%, of all NF cases. In the remaining cases, GABHS is most commonly isolated [6].
- aureus is a complex pathogen with primary infection ranging from simple folliculitis to systemic life-threatening disease [7-10]. Some strains contain virulent gene sets including Panton-Valentine-Leukocidin, enterotoxin gene, and antibiotic resistance [8-10].
Although previously unrecognized, NF involving a monomicrobial infection with S. aureus, typically methicillin-resistant S. aureus (MRSA), has now been defined [4- 8]. The incidence of monomicrobial infections with methicillin-sensitive S. aureus (MSSA) and MRSA has been increasing over the past two decades, yet it remains relatively low [5,6]. Furthermore, isolated S. aureus NF causing soft-tissue gas formation is exceedingly rare [4]. Therefore, we report on a case of indolent NF caused by a gas-producing strain of MSSA, occurring in a patient with previously undiagnosed type 2 diabetes mellitus (T2DM).
Day 0–3: A 46-year-old male with no previous medical, surgical, or social history presented to the emergency department (ED) with complaints of subjective fevers, chills, body aches, and non-bloody emesis for 3 days. He stated family members had recently experienced similar symptomatology. Vitals were within normal limits, and physical exam was unremarkable. All labs and viral panels were unremarkable. The patient was diagnosed with influenza-like illness and discharged. Day 6: He returned to the ED with intractable nausea, vomiting, and body aches. Triage labs showed serum glucose 500 mg/dL, hemoglobin (Hb) A1c 14.0%, lactic acid 2.8 mmoL/L, CO2 11 mmoL/L, and venous blood gas pH of 7.10. He was diagnosed with diabetic ketoacidosis (DKA) and admitted for medical management. The medical team hypothesized that the viral illness decompensated into DKA in the setting of undiagnosed T2DM.
Day 8: The patient was discharged home on Insulin Detemir and Aspart.
Day 11: Patient had a fall from standing at home onto his left knee without abrasions or lacerations.
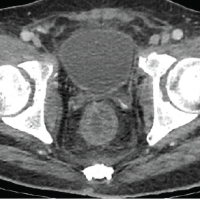
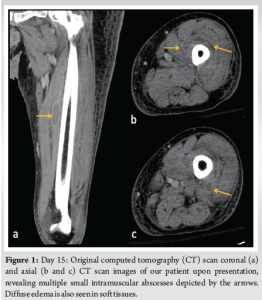
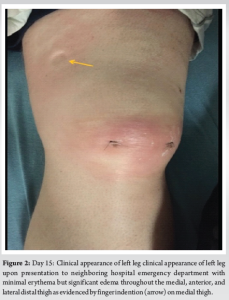
Day 15: He returned to the ED endorsing progressive pain, swelling, and weakness. The patient was AO×3, afebrile, toxic appearing, and tachycardic to 118 beats/min. Inspection of the left leg revealed faint erythema with warmth, with tenderness to palpation over the anterior mid-thigh to the mid-leg. The left thigh and leg compartments were soft and compressible. A small effusion was detected on examination of the left knee. The patient had limited range of motion to the left knee but was neurovascularly intact. The general surgery team was consulted for concern of a soft-tissue infection. Lab results revealed an elevated white blood cell count (21.6 K/mcL), erythrocyte sedimentation rate (90 mm/h), C-reactive protein (274 mg/L), and serum glucose (301 mg/dL). The patient had decreased serum sodium levels (123 mEq/L) and Hb (11.1 g/dL). The serum creatinine was within the normal range (0.96 mg/dL). Laboratory Risk Indicator for NF (LRINEC) score was 9, which was considered high risk for a developing NF [11-13]. Blood cultures drawn in the ED revealed MSSA bacteremia. Computed tomography (CT) of the left lower extremity revealed small pockets of peripheral enhancing fluid collections within the vastus medialis, intermedius, and lateralis consistent with multiple abscesses. The patient’s imaging also exhibited diffuse soft-tissue thickening and fat stranding consistent with cellulitis (Fig. 1).
The general surgery team elected to treat the patient medically with broad-spectrum intravenous (IV) antibiotics for pyomyositis. Orthopedics was consulted to rule out septic arthritis of the knee. Left knee arthrocentesis revealed 20 cc of clear synovial fluid, 3156 total nucleated cells, no crystals, and 86% polymorphonuclear leukocytes. Concern for septic arthritis was low. Day 17: Despite the low synovial fluid cell count, the left knee aspirate culture grew MSSA. Although the aspirate culture could have been a contaminant given his bacteremia, the patient continued to have a limited range of motion and pain. Consequently, the orthopedic team recommended a knee irrigation and debridement (I and D) for septic arthritis. After discussing the risks and benefits of both arthroscopic and open arthrotomy I and D procedures, the patient chose to an arthroscopic I and D during which cloudy joint fluid was seen. Intraoperative cultures grew MSSA. Day 19: The infectious disease team recommended continued IV cefazolin infusions, but the patient refused further treatment and left against medical advice.
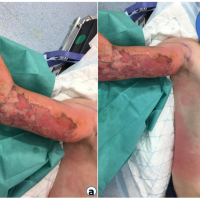
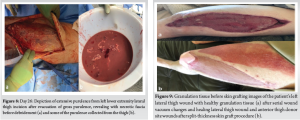
Day 26: The patient presented to the ED stating worsening appearance and pain in the left leg. The left leg had significant induration and swelling, but minimal erythema around his previous arthroscopic portal incisions, and no expressible purulent drainage (Fig. 2).
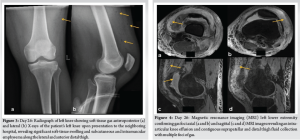
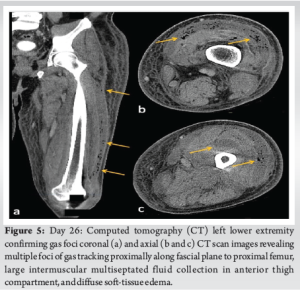
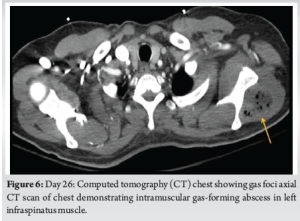
 Radiographs of the knee revealed distal thigh subcutaneous and intramuscular emphysema (Fig. 3). MRI of the knee revealed a knee effusion, distal thigh fluid collections, and significant gas foci (Fig. 4). CT of the thigh also exhibited large multiseptated fluid collections in the anterior compartment of the thigh, gas to the level of the greater trochanter along fascial planes, a knee effusion, and diffuse soft-tissue edema (Fig. 5). LRINEC score was 9 [11-13]. The patient also complained of new-onset discomfort in the left shoulder and right posterior chest. A chest CT-scan revealed a gas-forming intramuscular abscess in his left infraspinatus muscle and a right posterior chest wall abscess without gas (Fig. 6).
Radiographs of the knee revealed distal thigh subcutaneous and intramuscular emphysema (Fig. 3). MRI of the knee revealed a knee effusion, distal thigh fluid collections, and significant gas foci (Fig. 4). CT of the thigh also exhibited large multiseptated fluid collections in the anterior compartment of the thigh, gas to the level of the greater trochanter along fascial planes, a knee effusion, and diffuse soft-tissue edema (Fig. 5). LRINEC score was 9 [11-13]. The patient also complained of new-onset discomfort in the left shoulder and right posterior chest. A chest CT-scan revealed a gas-forming intramuscular abscess in his left infraspinatus muscle and a right posterior chest wall abscess without gas (Fig. 6).
Urgent open I and D of the left lower extremity, shoulder, and chest were recommended. The patient was counseled about the risks and benefits of operative versus nonoperative management. The patient elected for an I and D of the thigh but refused invasive procedures elsewhere.
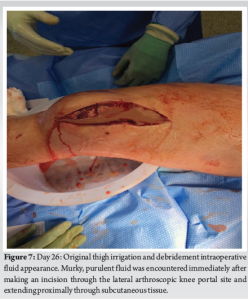
During the procedure, an incision was made over the anterolateral knee in line with the previous lateral scope portal incision. Significant purulence and “dishwater” fluid were present (Fig. 7). The incision was carried to the level of the greater trochanter. Blunt dissection along the fascial planes of the thigh showed complete dissociation of the fascia from the subcutaneous tissue. A thorough debridement was completed throughout the anterior thigh (Fig. 8). The NF traveled along the fascial plane to the level of the hip joint and inguinal ligament. An arthrotomy of the knee joint revealed clear synovial fluid without intraarticular purulence. Approximately 500 mL of purulent fluid was evacuated from the thigh. The involved musculature appeared grossly viable. Upon completion of the I and D, an irrigating wound vacuum-assisted closure (WV) dressing was placed over the thigh dead space and along the surgical wound. The irrigating wound vacuum delivered acetic acid for 24 h followed by normal saline.
Postoperatively, the patient refused additional operative interventions despite concerns for additional indolent soft tissue NF. Both the medicine and orthopedic team recommended repeat left thigh I and D with WV changes, right chest wall, and left infraspinatus I and D. The patient agreed to interventional radiology (IR)-guided drain placements for the chest and infraspinatus abscesses.
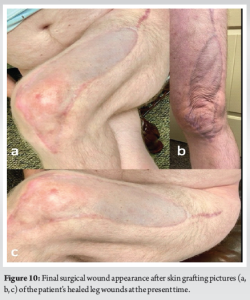
Day 29: The patient elected to undergo a repeat left thigh I and D and WV change. There was no evidence of muscle necrosis or purulence. Consequently, a regular WV was applied. All cultures from the thigh, shoulder, and chest wall again grew MSSA. The patient remained inpatient for 4 more days and received two bedside WV changes to the left thigh. Day 34: The patient refused further inpatient treatment and was discharged with home-health WV changes and IV antibiotics for 6 weeks. This was followed by a successful split-thickness skin graft (Fig. 9). Day 124: The patient continued to suffer from a persistent right chest wall gas-forming abscess. He received an additional IR-guided drain placement. Day 214: Persistent drainage from the wound forced an open I and D. The patient subsequently managed the wound with packing changes for 6 months before it healed by secondary intention. Although the patient’s posterior chest wall wound did not completely heal until 12 months after initial presentation, he was able to return to part-time employment at 3 months. And full-time employment at 5 months. His primary care provider and endocrinologist managed his diabetes with a combination of metformin and insulin. Aside from postoperative scar tissue formation, the patient states he has fully recovered, returned to all previous activities, and had no further necrotizing soft-tissue infections (Fig. 10).
- aureus as a single etiologic cause of NF is exceedingly rare, and coinciding gas production with MSSA is almost unheard of, with only one documented case in the available literature. Most NF cases, approximately 50%, are polymicrobial in nature, and single microorganism infections are almost always caused by Group A streptococcus or MRSA [4,5,7,8]. NF caused by S. aureus appears to be most common in middle-aged men, often with underlying T2DM [5,6]. In multiple studies with monomicrobial S. aureus, NF 33–44% of patients had T2DM [5,6]. DKA acutely preceded his progression to NF. Acute stressors, such as trauma, surgery, or infection can also increase the risk of DKA in patients with T2DM [14-16].
Many patients with NF decompensate rapidly, but diagnosing NF can be difficult early in the course of the infection [17]. Erythema is the most common symptom, but erythema, pain, warmth, induration, edema, or purulence occur in >95% of patients [17]. The most commonly affected areas appear to be the thigh and upper arm, though the presence of head-and-neck infection could hint at S. aureus as the cause [17,18]. Needle puncture, such as insulin injection, labs, or joint aspiration, may also predispose patients to a monomicrobial infection with S. aureus [7]. LRINEC scores historically have been used as an adjunctive score to help confirm suspicions of NF. The accuracy of these scores has been questioned in recent meta-analyses, however, with sensitivity being as low as 40.8% [19-26]. This is compounded in the diagnosis of more indolent presentations of NF, such as those caused by monomicrobial S. aureus. This patient initially presented with relatively mild symptoms, normal vitals, a high-risk LRINEC score, and indeterminate CT imaging concerning at the time for pyomyositis. Consequently, clinical suspicion for NF remained low on his first emergency room presentation. Only later, once additional imaging revealed multiple areas of gas did the elevated LRINEC score clinically correlate with a diagnosis of NF despite the patient’s continued clinical stability. We suspect his uncontrolled T2DM, multiple needle exposures, and MSSA bacteremia contributed to the development of an indolent and widespread infection with monomicrobial gas-forming MSSA [4- 8]. In the past, physicians often made medical decisions for patients, sometimes with little dialog [26-30]. Today, patients are expected to participate in shared decision-making [29]. While patient autonomy is paramount, encouraging patients to make medically sound decisions that optimize outcomes is an art form. Regrettably, this patient elected to forgo multiple medical teams’ recommendations and, at times, declined treatment altogether. This likely prolonged recovery and forced additional interventions. This patient’s mild symptomatology may have contributed to both providers’ difficulty guiding treatment and the patient’s insistence on minimizing interventions. Adopting a relationship-centered approach, enhancing autonomy with open dialog, and exploring the patient’s values before offering recommendations are evidence-based measures that may have improved compliance and outcomes in this case [29].
Although NF caused by monomicrobial infection with S. aureus has been reported in the literature, awareness of this condition remains limited, especially with concomitant gas formation. Most gas-producing necrotizing soft-tissue infections are polymicrobial in nature, and therefore, S. aureus as a single etiologic agent is exceedingly uncommon. Physicians should maintain a high index of suspicion for NF with S. aureus in patients with symptoms of early necrotizing soft-tissue infection, history of diabetes, or recent needle puncture. Mild presentation may complicate initial diagnosis and patient compliance. Regardless, a multidisciplinary care team should be utilized, all therapies, including non-surgical, should be considered, and providers should apply a relationship-centered approach and remain respectful of patient autonomy and optimize outcomes.
This case provides a visual and stimulating report on the importance of early clinical suspicion in the setting of atypical monomicrobial NF. It also stresses the importance of well-executed shared decision-making and its effects on outcomes.
References
- 1.Kao LS, Knight MT, Lally KP, Mercer DW. The impact of diabetes in patients with necrotizing soft tissue infections. Surg Infect (Larchmt) 2005;6:427-38. [Google Scholar | PubMed]
- 2.Lipsky BA, Berendt AR, Deery HG, Embil JM, Joseph WS, Karchmer AW, et al. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2004;39:885-910. [Google Scholar | PubMed]
- 3.Rajagopalan S. Serious infections in elderly patients with diabetes mellitus. Clin Infect Dis 2005;40:990-6. [Google Scholar | PubMed]
- 4.Saliba WR, Goldstein LH, Raz R, Mader R, Colodner R, Elias MS. Subacute necrotizing fasciitis caused by gas-producing Staphylococcus aureus. Eur J Clin Microbiol Infect Dis 2003;22:612-4. [Google Scholar | PubMed]
- 5.Cheng NC, Wang JT, Chang SC, Tai HC, Tang YB. Necrotizing fasciitis caused by Staphylococcus aureus: The emergence of methicillin-resistant strains. Ann Plast Surg 2011;67:632-6. [Google Scholar | PubMed]
- 6.Tsai YH, Wen-Wei Hsu R, Huang KC, Huang TJ. Comparison of necrotizing fasciitis and sepsis caused by Vibrio vulnificus and Staphylococcus aureus. J Bone Joint Surg Am 2011;93:274-84. [Google Scholar | PubMed]
- 7.Regev A, Weinberger M, Fishman M, Samra Z, Pitlik SD. Necrotizing fasciitis caused by Staphylococcus aureus. Eur J Clin Microbiol Infect Dis 1998;17:101-3. [Google Scholar | PubMed]
- 8.Morgan WR, Caldwell MD, Brady JM, Stemper ME, Reed KD, Shukla SK. Necrotizing fasciitis due to a methicillin-sensitive Staphylococcus aureus isolate harboring an enterotoxin gene cluster. J Clin Microbiol 2007;45:668-71. [Google Scholar | PubMed]
- 9.Lee YT, Chou TD, Peng MY, Chang FY. Rapidly progressive necrotizing fasciitis caused by Staphylococcus aureus. J Microbiol Immunol Infect 2005;38:361-4. [Google Scholar | PubMed]
- 10.Perbet S, Soummer A, Vinsonneau C, Vandebrouck A, Rackelboom T, Etienne J, et al. Multifocal community-acquired necrotizing fasciitis caused by a Panton-valentine leukocidin-producing methicillin-sensitive Staphylococcus aureus. Infection 2010;38:223-5. [Google Scholar | PubMed]
- 11.Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (laboratory risk indicator for necrotizing fasciitis) score: A tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med 2004;32:1535-41. [Google Scholar | PubMed]
- 12.El-Menyar A, Asim M, Mudali IN, Mekkodathil A, Latifi R, Al-Thani H. The laboratory risk indicator for necrotizing fasciitis (LRINEC) scoring: The diagnostic and potential prognostic role. Scand J Trauma Resusc Emerg Med 2017;25:28. [Google Scholar | PubMed]
- 13.Bechar J, Sepehripour S, Hardwicke J, Filobbos G. Laboratory risk indicator for necrotising fasciitis (LRINEC) score for the assessment of early necrotising fasciitis: A systematic review of the literature. Ann R Coll Surg Engl 2017;99:341-6. [Google Scholar | PubMed]
- 14.Newton CA, Raskin P. Diabetic ketoacidosis in type 1 and type 2 diabetes mellitus: Clinical and biochemical differences. Arch Intern Med 2004;164:1925-31. [Google Scholar | PubMed]
- 15.Wang Y, Desai M, Ryan PB, DeFalco FJ, Schuemie MJ, Stang PE, et al. Incidence of diabetic ketoacidosis among patients with type 2 diabetes mellitus treated with SGLT2 inhibitors and other antihyperglycemic agents. Diabetes Res Clin Pract 2017;128:83-90. [Google Scholar | PubMed]
- 16.Kitabchi AE, Umpierrez GE, Murphy MB, Kreisberg RA. Hyperglycemic crises in adult patients with diabetes: A consensus statement from the American Diabetes Association. Diabetes Care 2006;29:2739-48. [Google Scholar | PubMed]
- 17.Ogilvie CM, Miclau T. Necrotizing soft tissue infections of the extremities and back. Clin Orthop Relat Res 2006;447:179-86. [Google Scholar | PubMed]
- 18.Brook I, Frazier EH. Clinical and microbiological features of necrotizing fasciitis. J Clin Microbiol 1995;33:2382-7. [Google Scholar | PubMed]
- 19.Hsiao CT, Chang CP, Huang TY, Chen YC, Fann WC. Prospective validation of the laboratory risk indicator for necrotizing fasciitis (LRINEC) score for necrotizing fasciitis of the extremities. PLoS One 2020;15:e0227748. [Google Scholar | PubMed]
- 20.Abdullah M, McWilliams B, Khan SU. Reliability of the laboratory risk indicator in necrotising fasciitis (LRINEC) score. Surgeon 2019;17:309-18. [Google Scholar | PubMed]
- 21.Neeki MM, Dong F, Au C, Toy J, Khoshab N, Lee C, et al. Evaluating the laboratory risk indicator to differentiate cellulitis from necrotizing fasciitis in the emergency department. West J Emerg Med 2017;18:684-9. [Google Scholar | PubMed]
- 22.Fernando SM, Tran A, Cheng W, Rochwerg B, Kyeremanteng K, Seely AJ, et al. Necrotizing soft tissue infection: Diagnostic accuracy of physical examination, imaging, and LRINEC score: A systematic review and meta-analysis. Ann Surg 2019;269:58-65. [Google Scholar | PubMed]
- 23.Waness A. Severe gas-forming necrotizing fasciitis of the right hip and thigh: Case presentation and review. Clin Case Rep Rev 2016;2:562-4. [Google Scholar | PubMed]
- 24.Misiakos EP, Bagias G, Patapis P, Sotiropoulos D, Kanavidis P, Machairas A. Current concepts in the management of necrotizing fasciitis. Front Surg 2014;1:36. [Google Scholar | PubMed]
- 25.Fisher JR, Conway MJ, Takeshita RT, Sandoval MR. Necrotizing fasciitis. Importance of roentgenographic studies for soft-tissue gas. JAMA 1979;241:803-6. [Google Scholar | PubMed]
- 26.Emanuel EJ, Emanuel LL. Four models of the physician-patient relationship. JAMA 1992;267:2221-6. [Google Scholar | PubMed]
- 27.Szasz TS, Hollender MH. A contribution to the philosophy of medicine; the basic models of the doctor-patient relationship. AMA Arch Intern Med 1956;97:585-92. [Google Scholar | PubMed]
- 28.Perry CB, Applegate WB. Medical paternalism and patient self-determination. J Am Geriatr Soc 1985;33:353-9. [Google Scholar | PubMed]
- 29.Quill TE, Brody H. Physician recommendations and patient autonomy: Finding a balance between physician power and patient choice. Ann Intern Med 1996;125:763-9. [Google Scholar | PubMed]
- 30.Fowler VG Jr., Sanders LL, Sexton DJ, Kong L, Marr KA, Gopal AK, et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: Experience with 244 patients. Clin Infect Dis 1998;27:478-86. [Google Scholar | PubMed]