Patients with end-stage renal disease are at increased risk for spontaneous femoral neck fractures due to renal osteodystrophy, even in the absence of major trauma. The presence of rare blood group antibodies, such as those in the Cromer system, can significantly complicate transfusion strategies and require specialized hematologic coordination.
Dr. Rahul Mittal, Department of Health Informatics, Rutgers - School of Health Professions, Piscataway, New Jersey. E-mail: rm1703@shp.rutgers.edu
Introduction: Bilateral displaced femoral neck fractures are uncommon in adults without significant trauma, especially in the absence of high-energy impact. This case highlights the importance of considering underlying metabolic disorders such as end-stage renal disease (ESRD) as significant contributing factors. It is one of the few reported cases demonstrating the interplay between ESRD, renal osteodystrophy, anemia of chronic disease, and the presence of rare Cromer blood group antibodies, adding a unique dimension to orthopedic and hematological literature.
Case Report: We present the case of a 50-year-old African American female with a history of ESRD on dialysis, who presented with bilateral hip pain after a minor fall. Imaging revealed bilateral displaced femoral neck fractures. Laboratory workup showed a hemoglobin level of 7.4 g/dL and microcytic anemia. Due to her chronic kidney disease, she was diagnosed with renal osteodystrophy, contributing to bone fragility. A rare Cromer blood group antibody was also detected, complicating transfusion planning. She underwent surgical repair of both hips, and her post-operative management involved coordinated multidisciplinary care addressing orthopedic, hematologic, and nephrologic concerns.
Conclusion: This case underscores the need for clinicians to consider renal osteodystrophy in patients with ESRD as a potential cause of spontaneous or low-impact fractures. In addition, it highlights the transfusion challenges posed by rare blood group antibodies like those in the Cromer system. The case demonstrates the value of early recognition, prompt surgical intervention, and interprofessional collaboration in managing complex comorbid conditions. It contributes to broader clinical understanding by linking metabolic bone disease, chronic anemia, and immunohematologic complications in fracture management. Further research into personalized treatment protocols and rare blood antigen matching could enhance outcomes for such medically complex patients.
Keywords: Renal osteodystrophy, bilateral femoral neck fracture, anemia of chronic disease, end-stage renal disease, Cromer blood group antibody.
Total hip arthroplasty (THA) is commonly performed in patients with end-stage osteoarthritis and chronic pain unresponsive to conservative treatment, with over 280,000 procedures annually in the U.S [1]. As life expectancy rises, THA utilization is expected to increase [2]. While osteoarthritis is the primary indication, conditions such as inflammatory arthritis, dysplasia, malignancy, and metabolic bone diseases also necessitate THA. Renal osteodystrophy, a complication of chronic kidney disease (CKD), arises due to impaired regulation of calcium and phosphate, leading to poor bone mineralization and increased fracture risk [3,4]. Up to 100% of patients with a glomerular filtration rate <60 mL/min may develop bone disease [3]. Musculoskeletal issues in CKD patients contribute to reduced mobility and increased morbidity. Although exercise improves outcomes, pain and fracture risk often limit physical activity [5]. Given that over 325,000 U.S. patients and 1.2 million worldwide are on dialysis, orthopedic management of pathological fractures is increasingly common [4]. Blood transfusions are frequent in THA, with rates from 18% to 68% [6]. However, restrictive transfusion practices are preferred due to risks such as sepsis, pneumonia, and thromboembolism [7]. Pre-operative anemia, seen in 15–33% of TJA patients, is associated with higher infection rates, longer hospital stays, and increased mortality [8,9]. This case highlights a patient with end-stage renal disease (ESRD) and bilateral hip fractures following minor trauma. Diagnosed with renal osteodystrophy, the patient was planned for THA but was found to have anemia and a rare Cromer antibody, complicating transfusion needs and contributing to post-operative encephalopathy.
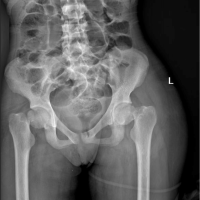
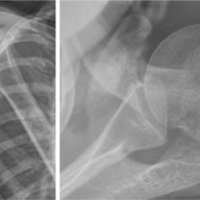
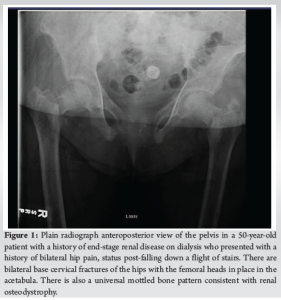
A 50-year-old female with a past medical history of ESRD on dialysis, coronary artery disease (CAD), hypertension, and dyslipidemia presented to the emergency department with bilateral hip pain after she had sustained a fall while at home just before arrival. Per Emergency Medical Services, the patient fell down a full flight of stairs. The patient states that she felt a “pop” with subsequent pain in the hips and has been unable to move her hips since. On physical examination, the patient was in no acute distress and was awake, alert, and oriented ×3 with no evidence of altered mental status. She had appropriate tenderness over the hips bilaterally. The patient was neurologically and vascularly intact with palpable dorsalis pedis pulses, warm toes, and intact sensation. An anteroposterior (AP) view X-ray of the pelvis (Fig. 1) and frog-leg lateral views of each hip individually were ordered. Universal mottled bone patterns consistent with renal osteodystrophy/secondary hyperparathyroidism with erosive and destructive changes on both sides of the pubic symphysis were visualized. Bilateral basicervical fractures of the hips were also noted. The patient also had a computerized tomography (CT) scan without contrast of the pelvis, which revealed acute transcervical fractures through the proximal femurs bilaterally and an acute displaced fracture of the left greater trochanter. Severe degenerative changes at the pubic symphysis with probable brown tumors in the pubic bone and L5 and S1 vertebral bodies were also noted, which were consistent with the X-ray findings. The patient also had a CT of the head without contrast performed, which did not reveal any significant findings.
Based on these findings, the patient was consented for bilateral hip arthroplasties as a means of treatment due to her history of ESRD and metabolic bone disease, which led to bilateral pathological fractures of the hip. During the routine blood screening prior to surgery and during admission, it was discovered that the patient had hemoglobin (Hb) of 7.4, a mean corpuscular volume of 74, and was fecal occult blood test positive. She had no history of prior blood transfusions. The patient also tested positive for a rare alloantibody, a Cromer antibody, which was detected during the routine blood screening before surgical intervention No specific symptoms prompted further specialized testing beyond this pre-operative screening. On her 2nd day of admission, her Hb was at 7.8, and her blood was drawn and sent out to the Red Cross blood bank to search for compatible blood to be given before surgery. The patient refused autologous transfusion due to the risk of self-rejection, as she had a history of antibody-mediated sensitization and likely would have rejected her own salvaged blood intraoperatively. Local blood banks and the American Red Cross were contacted but were unable to provide compatible blood due to the patient’s rare blood antibody. As surgery was not emergent, the patient was rescheduled until blood products were available. In the interim, the patient was managed with Aranesp 200 mcg once weekly with dialysis, Venofer 200 mg once daily with a total of 5 doses, and folic acid 1 mg PO daily. Eventually, 2 units of frozen blood were located in Wisconsin and delivered to the hospital, knowing that the risk of hemolysis was significant. Risks were discussed with the patient, and the patient consented to having transfusions to proceed with her surgery. 1 week before surgery, the patient was transfused with 1 unit of blood given her history of CAD. A few days later, after receiving the transfusion, the patient suffered from uremia-induced encephalopathy and was unable to give consent, obligating her son to provide consent. The patient’s blood urea nitrogen was noted to be 67 mg/dL. The patient’s labs from that day can be seen in (Fig. 2). Despite decubitus precautions, the patient also suffered from decubitus ulcers of the sacrum. Multiple family meetings involving the nursing staff, hematology service, orthopedic surgery service, and social work resulted in the patient and her family agreeing to have the surgery, given the risks of bleeding, hemolysis, shock, and other life-threatening conditions. The morning of surgery, the patient was dialyzed and given the second unit of blood. The night before surgery, the patient’s Hb was 7.4 mg/dL.

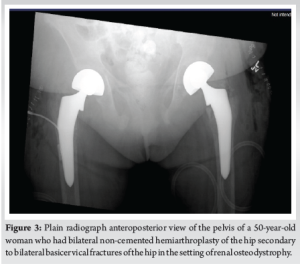
28 days after admission and initial presentation, the patient consented to bilateral non-cemented hemiarthroplasties performed through the direct anterior approach with the patient supine. The surgeon felt that less patient movement in this approach (as compared to the lateral decubitus position) would make the surgery more efficient due to decreased blood loss and a lack of available blood products. On the right, a size 7 collared stem minus head with a 51 bipolar component was used. On the left, a size 7 collated stem plus 0 head with a 52 bipolar component was used. Both implants were Medacta. Bilateral release of hip contractures with capsulotomies with removal of multiple bone fragments was performed. Femoral head bone biopsies were also taken. The tensor fascia latae was closed with a #1 Vicryl followed by 0 Vicryl staples. A Prevena vacuum dressing was applied with excellent suction. There were no complications during the procedure as the patient was successfully extubated and transferred to the recovery room. On post-operative day 7, the patient was able to bear weight after sitting at the edge of the bed. The patient reported 8/10 right knee pain but could not give a numerical value for back pain. The patient agreed to participate in physical therapy upon discharge. Post-operative AP X-rays of the pelvis can be seen in (Fig. 3).
During her first post-operative clinic visit, the patient was alert and oriented, ambulating with a front-wheeled walker under supervision. On physical examination, the surgical wounds were clean and dry without signs of infection. She reported reduced pain in the hips and improved mobility compared to her pre-operative status. No dislocations or implant-related complications were noted on follow-up radiographs. The patient expressed satisfaction with the outcome and was referred for outpatient physical therapy to continue rehabilitation. Laboratory studies at follow-up showed stable Hb levels and improved renal parameters with dialysis. The patient’s last known follow-up was approximately 6 months post-operative. The patient resided in a nursing home, demonstrated improved mobility, and maintained stable prostheses with no reported complications.
Bilateral displaced femoral neck fractures are rare in adults with normal bone density, usually resulting from major trauma such as vehicle accidents or high falls [10,11]. However, patients with metabolic conditions like ESRD are more prone to these fractures due to renal osteodystrophy, which alters bone metabolism. Renal osteodystrophy arises from impaired glomerular filtration, causing phosphate retention and hyperphosphatemia. Elevated phosphate reduces 1,25-dihydroxyvitamin D levels, limiting calcium absorption. The resulting hypocalcemia stimulates parathyroid hormone production, leading to increased bone resorption and osteoporosis [3,4,12,13]. This process likely contributed to the fractures in our patient, an older female with ESRD. A similar case was reported by Sathyanarayana et al., where a patient with renal osteodystrophy sustained bilateral subcapital hip fractures. Studies show that patients with renal osteodystrophy have a significantly higher risk of such fractures and a 2.5-fold increase in 1-year mortality following hip fractures compared to the general population [14,15]. Surgical intervention, combined with aggressive mobilization, has shown better outcomes than conservative treatment in terms of mortality and complications [16,17]. This patient also presented with low Hb and microcytic anemia, suggestive of anemia of chronic disease, which is often linked to conditions such as cancer, infection, or autoimmune disease [18]. In ESRD, anemia is mainly due to erythropoietin deficiency and the suppressive effects of uremic toxins on red blood cell production. Chronic inflammation from dialysis or recurrent infections may exacerbate this condition [19]. Restoring Hb to near 12 g/dL improves quality of life for patients with renal failure [20]. Transfusions are typically warranted when Hb levels fall below 8.0 g/dL, or below 6.5 g/dL in life-threatening cases [19]. Our patient, with a Hb of 7.4 g/dL, met criteria for transfusion but had a rare complicating factor: An uncommon blood group antibody. The Cromer blood group system includes antigens located on decay-accelerating factor, a membrane protein involved in complement regulation. First reported in 1965 by McCormick et al., the anti-Cromer (Cra) antibody was identified in a patient initially thought to have anti-Gob. Later work revealed that it was a distinct antibody, now recognized within the Cromer system [21]. These antibodies are rare and mostly found in individuals of African descent, often stimulated by pregnancy. There are 19 known Cromer antigens – 16 high incidence and three low incidence – many associated with specific ethnic groups [22,23]. For example, Tca was discovered in two Black females with no transfusion history, while anti-Dra is mostly seen in Bukharan Jews [21,24]. Antibodies against high-frequency Cromer antigens, though rare, can complicate transfusions and may accelerate the destruction of transfused red blood cells [21]. This patient’s rare antibody posed a serious challenge in sourcing compatible blood. Such cases highlight the need for specialized transfusion strategies and donor databases for rare blood types. Managing this case required close coordination across specialties – hematology, nephrology, orthopedics, and transfusion medicine. From diagnosis through post-operative care, multidisciplinary collaboration ensured comprehensive treatment, illustrating the value of interprofessional teamwork in complex cases.
Continued research into the immunogenetics of rare blood group systems, such as the Cromer antigens, is essential for developing novel transfusion strategies and improving donor compatibility for sensitized patients. Finally, promoting interdisciplinary collaboration among hematologists, orthopedic surgeons, nephrologists, and transfusion medicine specialists is crucial for developing comprehensive care pathways that address both the fracture management and the complex medical needs of patients with ESRD. In cases like these, where multiple medical issues are intertwined, ranging from bone metabolism disturbances and anemia of chronic disease to the complications associated with rare blood group antibodies, collaborative teamwork becomes indispensable. This collaborative effort not only ensures that all aspects of the patient’s condition are addressed but also enhances decision-making, reduces errors, and improves overall patient outcomes. By promoting interprofessional education and collaboration, healthcare teams are better equipped to navigate the complexities inherent in managing patients with multiple, overlapping health challenges.
This case represents a complex array of clinical problems that required a multi-disciplinary approach involving the nursing staff, social work, physical therapy, medicine service, nephrology service, hematology service, cardiac service, and orthopedic Surgery service to coordinate proper care and management for this patient. It provides an example of how modern medicine has adapted a team-based approach to provide the best course of treatment for the patient.