Combined approach is a middle path regimen for GCTB of the proximal femur with joint involvement.
Dr. Sunil D Magadum, Department of Orthopaedics, MIOT International Hospital, Chennai, Tamil Nadu - 600089, India. E-mail: mobility@miotinternational.com
Introduction: Giant cell tumor of the bone (GCTB) is a locally aggressive benign tumor involving the ends of the long bones seen in the second or third decade of life, more commonly in women. Even though it is benign, it has a potential for malignant transformation in 10% of cases and metastasis to the lung in 1–4% of patients. GCTB of the proximal femur has a high recurrence rate, high incidence of pathological fracture, and poor prognosis.
Case Report: A 29-year-old man presented with pain and swelling in the left proximal femur of 1 year duration. After confirmation of his diagnosis using fine-needle aspiration cytology, he underwent a combined approach, i.e., near total excision of the tumor, extended curettage of the remaining portion of the tumor, and total hip replacement, followed by systemic therapy using monthly injection of denosumab for 1 year.
Conclusion: At 2½ years of follow-up, there was no evidence of recurrence of tumor either clinically or radiologically. Patient’s pre-operative Harris Hip score was 20, which was improved to 92 at the latest follow-up. A combined approach using surgery and systemic therapy using denosumab can be considered in the proximal femoral giant cell tumor.
Keywords: Giant cell tumor of bone, extended curettage, segmental resection, total hip replacement, denosumab.
Giant cell tumor of the bone (GCTB) is a locally fast-growing, aggressive tumor that commonly involves the ends of long bones. According to various studies, GCTB is responsible for 20% of all benign bone tumors, with malignant transformation occurring in about 10% of GCTB and lung metastasis occurring in 1–4% of patients. They affect mainly the young adults aged between 20 and 40 years, more common in women, with a high local recurrence rate (12–55%) [1]. The prognosis of giant cell tumor (GCT) also depends on the anatomical location of the tumor, and oncological complications are more frequently seen in proximal femoral GCT [2]. Histologically, GCTB consists of proliferating stromal cells intermixed with numerous osteoclast-type giant cells, a combination that produces its characteristic osteolytic behavior [3]. Despite being regarded as benign, these tumors can exhibit erratic behavior, such as soft tissue expansion, cortical destruction, local recurrence following curettage, and, in rare instances, pulmonary metastases. Proximal femoral involvement is relatively uncommon, comprising about 5.5% of GCT cases. Because such lesions often involve the neck and intertrochanteric region – areas subject to considerable biomechanical stress – management is particularly demanding [4]. These lesions are usually seen in the femoral neck and intertrochanteric area; however, occasionally, they involve the hip joint through femoral head penetration, affecting the function of the hip. This site is also common for pathological fractures in view of the destruction of the bone due to the pathological process in delayed presentation, and also due to the high stress area in this zone. Hence, the treatment of the GCT in the proximal femur is challenging [5]. The treatment options for proximal femoral GCTs are extended curettage (EC), with or without filling the cavity with bone graft or cement, segmental resection (SR), and reconstruction using a tumor hip prosthesis. EC has disadvantages such as a higher rate of local recurrence, osteonecrosis of the femoral head with secondary osteoarthritis, and potential morbidity for future surgery; however, it has the advantage of preserving the joint. SR and reconstruction using tumor hip prosthesis has the advantage of a low recurrence rate, but it has disadvantages such as infection, limited prosthesis survival, and poor joint function, especially in younger patients [6]. Denosumab which is a human monoclonal antibody that acts against the receptor activator of nuclear factor kappa-B ligand (RANKL) and inhibits the RANKL pathway has been advocated for systemic use of GCTB. It is usually used as a neo-adjuvant agent to reduce the pain, decrease the morbidity of the surgical procedure, to get consistent radiological changes, and to reduce the size of the tumor in inoperable cases [7]. Here, we are presenting a rare case report highlighting the importance of a combined approach (i.e., surgery and systemic therapy with injection denosumab) in a 29-year-old man with a GCT of a proximal femur who underwent near-total excision of the tumor, reconstruction using a total hip replacement (THR) prosthesis, and systemic therapy with denosumab.
A 29-year-old man from South India presented with pain in the left hip for the past 1 year, with increased intensity for the past 5 months. Pain was dull aching, continuous without any diurnal variation. He also has difficulty walking for the past 5 months. On examination, there was tenderness around the left trochanteric region and around the joint line. His movements of the hips were restricted and extremely painful. He was primarily wheelchair-bound with limited mobility. His X-ray and computed tomography (CT) scan showed an eccentric expansile lytic lesion measuring approximately 7.7 × 5.7 × 3.2 cm in the proximal left femur involving the superolateral portion of the femoral head, neck of femur, greater trochanter and intertrochanteric region (H1 and H2 area and minimal involvement of H3 area as per the International Society of Limb Salvage) with no matrix mineralization [Fig. 1 and 2].
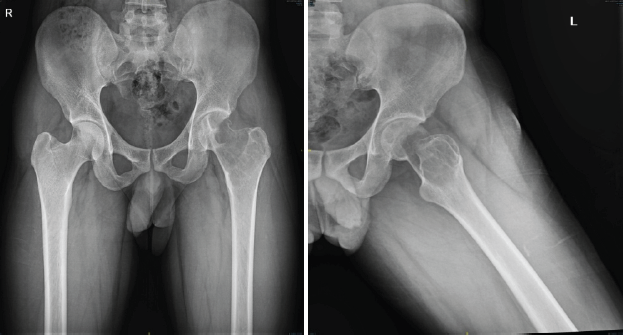
Figure 1: X-ray pelvis shows large lytic cavity in the femoral head, neck, and trochanteric area of the left proximal femur. These are pre-operative X-rays taken before the surgical procedure, at the time of presentation.
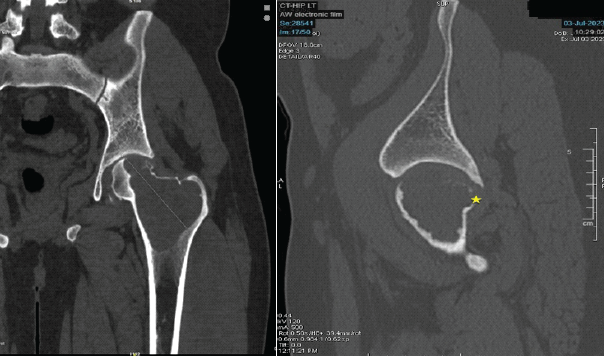
Figure 2: Computed tomography scan shows extent of involvement with breach in the superior cortex portion of femoral head and neck. These are pre-operative computed tomography scan images taken before the surgical procedure, at the time of presentation. These sections confirm the bony involvement and extent of the lytic lesion, essential for pre-operative planning.
Cortical dehiscence was noted in the superolateral aspect of the femoral head and also in the anterior and posterior cortices of the neck of the femur with mild effusion in the hip joint. His magnetic resonance imaging reports also confirmed similar findings with narrow zones of transition without acetabular involvement. His routine blood chemistry and chest CT scan were normal. He underwent ultrasound-guided fine-needle aspiration cytology, which confirmed the diagnosis of GCT. The case was discussed in tumor board meeting. Since there was an extensive involvement of the femoral head and neck, and trochanteric area with a cortical break in the femoral head and neck, he was planned for near total excision of the tumor, EC of the remaining lesion from the lesser and greater trochanteric area, and THR, along with systemic therapy using injection denosumab.
Surgical technique
Patient in right lateral position, posterior approach to the left hip was used. Hip joint exposed and dislocated posteriorly. A vertical cut was made over the medial border of the trochanter, leaving the greater trochanter attached to the main shaft. A horizontal cut was made at the level of the lesser trochanter, and the entire femoral head, neck, and part of the trochanter was removed. The remaining portion of tumor around the medial border of the greater and lesser trochanter was curetted thoroughly using curettes and a high-speed burr, and flushed with a mixture of saline and hydrogen peroxide. Utmost precaution was taken to avoid the spillage of the tumor [Fig. 3]. Samples were sent for histopathology [Fig. 4].
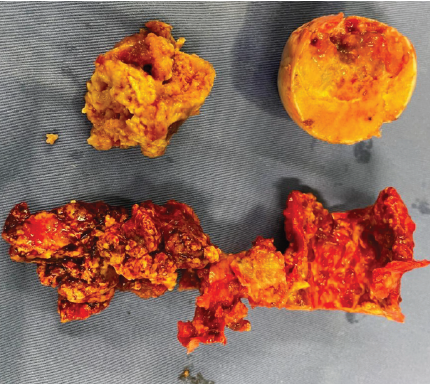
Figure 3: Intraoperative photograph showing tumor tissue and femoral head with defect. This image was taken in the intraoperative period, following the surgical procedure in the operation theater.
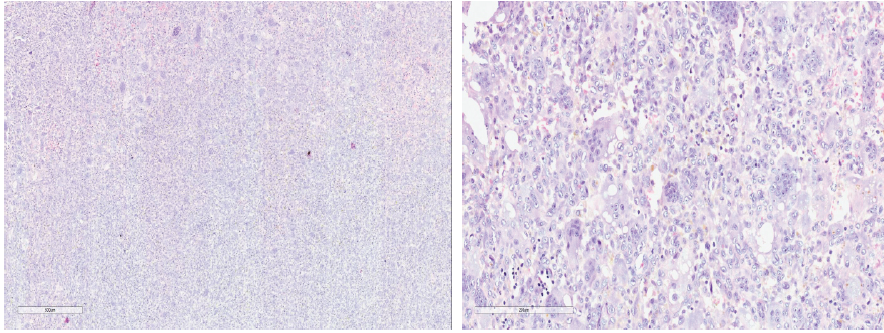
Figure 4: ×50 and ×200 Magnification showing multinucleated giant cells and mononuclear cells suggestive of giant cell tumor. These are microscopic images showing multinucleated giant cells and mononuclear cells (which are suggestive of giant cell tumor). The samples were taken from the lytic lesion in the left proximal femur.
The acetabular cartilage was normal without any tumor involvement. Preparation for the acetabulum was done and dual mobility acetabular shell of size 51 was inserted. On the femoral side, after preparation of the femoral canal, a modular hip stem (SROM) size 18 × 13 calcar replacement standard stem and femoral sleeve 18F was used. Acetabular liner of corresponding size (51 × 28 mm) and femoral head 28/0 was locked using a locking mechanism, and hip joint was reduced. Hip joint was found to be stable in all directions [Fig. 5].
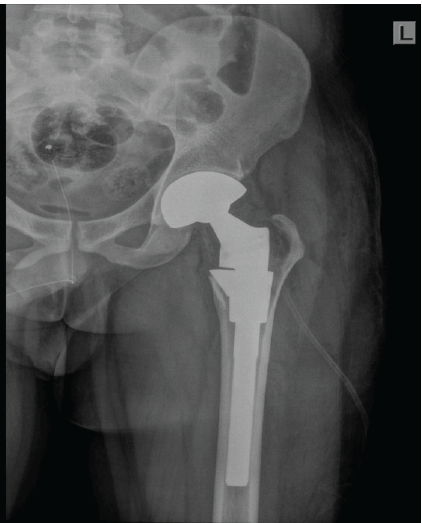
Figure 5: Immediate post-operative X-ray shows well positioned total hip replacement prosthesis with post curettage defect in the medial border of greater trochanter.
Post-operative care
The patient was mobilized with partial weight bearing with the help of two crutches for 1 month followed by full weight-bearing walking with support. After 2 months, he was made to walk without support. Quadriceps dynamic and static exercises started from day 1. Low molecular weight heparin was used as an anticoagulant for 1 week followed by oral antiplatelet drugs for 2 months. The first dose of injection denosumab 120 mg was given subcutaneously 1 week after surgery and continued monthly for 1 year. A venous Doppler study was done at the time of discharge to rule out deep vein thrombosis. His post-operative histopathology report has confirmed the diagnosis of GCT. The patient was followed up monthly for 3 months, 6 months, 1 year, and further follow-up to date to observe the recurrence of the tumor and improvement in his hip function.
The patient is walking full weight bearing without support and is able to carry out all his daily activities without any discomfort. Patient’s pre-operative Harris hip score (HHS) was 20, which was improved to 86 at the latest follow-up. There was no evidence of recurrence of tumor noted clinically and radiologically [Fig. 6].
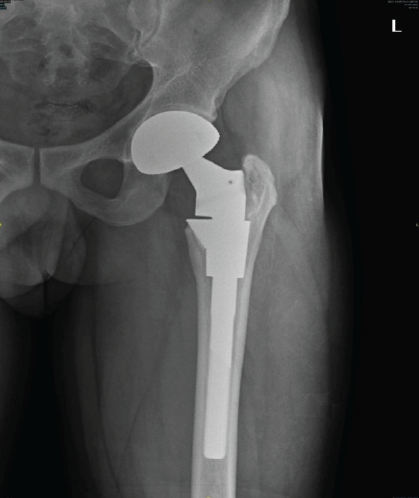
Figure 6: 2 year follow-up X-ray showing no evidence of any loosening and ossification in medial border of trochanter.
GCT of the bone is one of the most commonly discussed tumors with a lot of controversies ranging from its etiology to its management. The exact histogenesis of GCT is not well known, and the correlation between its histology and its clinical course is undefined [8,9,10,11]. Even though Campanacci’s classification is used to grade the tumor, many studies have demonstrated no correlation between Campanacci grade of the tumor and the risk of local recurrence or metastasis, hence it cannot be used a single prognostic factor [12]. There are differences of opinion regarding the exact management, with many authors recommending achieving a proper local control and maintaining the function. Hence, EC using high-speed grinding and drilling to remove the invaded bone has been recommended. Adjuvants such as phenyl alcohol, iodine, hydrogen peroxide, or zinc chloride have been used by many authors to reduce the post-operative recurrence rate [13,14]. These adjuvants help to remove the tumor cells which were left behind after the curettage either by their thermal (liquid nitrogen, methyl methacrylate) or by chemical reaction (phenyl alcohol, H2O2). There is also a difference of opinion regarding filling the cavity after EC. Kivioja et al. in their study about the use of cement after intralesional curettage in GCT in 294 patients with a median follow-up of 5 years had shown a 0.2% recurrence rate with cement and 0.56% without cement. They recommended the use of cement as a good prognostic factor [8]. On the contrary, Turcotte et al. in their 186 patients, found no statistical significance between the use of adjuvant and filling material with the risk of local recurrence [10]. Some studies even reported thermal necrosis of the articular cartilage and non-fusion of the cement subchondral interface after use of cement. Hence, allogenic bone transplantation (3 mm–10 mm thickness) was advocated, and it is soaked in H2O2 to remove its immunogenicity [15]. GCT of the proximal femur has low incidence, high recurrence rate after curettage, and more local invasiveness with strong bone destructiveness leading to pathological fracture [16]. EC or SR is the two most commonly used procedures for these tumors. The use of high-speed burr in EC in tumors around the femoral neck is difficult because of thin quality of bone due to tumor destruction and may lead to increased risk of pathological fracture [17]. On the other hand, SR and reconstruction usually result in poor functional outcome and increased risk of complication hence it is difficult to decide whether to do SR and reconstruction to minimize the local recurrence versus to preserve the joint with a more conservative approach [18,19]. Yuan et al. did a comparative analysis between EC and SR in GCT of the proximal femur, showed 5.3% recurrence rate in EC group and 10% in SR group in 29 patients. They recommended EC in patients without extensive soft tissue and articular surface involvement and patients without pathological fracture for better functional outcome and survival. In other cases, SR is recommended. Denosumab inhibits RANKL pathway, which in turn inhibits osteoclast activation and prevents osteolysis. After denosumab treatment mononuclear cells and giant cells disappear, there is partial maturation of neoplastic stromal cells to osteoblastic cells and fibrous cells, which also helps to form osteoid matrix. Denosumab helps to reduce the size of the tumor, forms calcified ream around the soft-tissue components, thus helping to do a local control, allowing EC with adjuvants or en bloc resection with endoprosthetic replacement possible in previously unresectable tumors. It is also indicated in the treatment of axial bone GCT, where surgery is difficult. Hence, the use of denosumab has been recommended as a neoadjuvant agent [12]. Its use has been approved by the Food and Drug Administration in 2013 for patients with GCT who are either inoperable or surgery may cause unacceptable morbidity, or in patients with metastatic disease. In our patient, the extent of involvement of the tumor was primarily in H1 and H2 areas with minimal involvement in H3 area in the left proximal femur with cortical dehiscence in the superolateral aspect of the femoral head and anterior and posterior cortices of the femoral neck. Patient’s mobility was also limited for the past 5 months. EC was not considered as a treatment option due to the extent of involvement, breach in the femoral head-and-neck cortices, and this area being prone to high recurrence rate and pathological fracture. SR was not considered since the patient was young, and this procedure has a high complication rate and poor functional outcome in the long term. Hence, it was decided to use a combined approach, i.e., near total excision of the tumor, EC of the remaining portion of the involved area of greater and lesser trochanter, and THR using hip prosthesis, followed by systemic therapy using injection denosumab 120 mg subcutaneously postoperatively once a month for 12 months. Denosumab injection helped in the ossification of the curetted area around the trochanteric region. At the end of 3 years of follow-up, there is no evidence of any recurrence of the tumor with improved patient’s functional outcome (patient’s pre-operative HHS was 20, which was improved to 86 at the latest follow-up). Hence, this combined approach of local clearance of the tumor by surgery and systemic adjuvant denosumab injections helped us to achieve a successful outcome. However, a complete prospective case series with long-term follow-up will throw more light on the usefulness of this procedure.
GCTB is a benign locally aggressive tumor with a high incidence of local recurrence, has potential for malignant transformation, and rarely metastasis to lungs. GCT of the proximal femur has a high incidence of local recurrence and pathological fracture. EC can be considered in patients without joint and soft tissue involvement, in patients without a pathological fracture. SR and endo-prosthetic replacement have a low incidence of recurrence; however, they have a poor functional outcome and high systemic morbidity. Hence, we conclude that a combined approach using local possible clearance of the tumor by surgery and systemic adjuvant therapy with injection denosumab postoperatively is a useful mid-path regimen in the management of GCT of the proximal femur.
GCTB of the proximal femur requires meticulous planning to get a successful outcome.
References
- 1. Niu X, Zhang Q, Hao L, Ding Y, Li Y, Xu H, et al. Giant cell tumor of the extremity: retrospective analysis of 621 Chinese patients from one institution. J Bone Joint Surg Am 2012;94:461-7. [Google Scholar] [PubMed]
- 2. Errani C, Ruggieri P, Asenzio MA, Toscano A, Colangeli S, Rimondi E, et al. Giant cell tumor of the extremity: A review of 349 cases from a single institution. Cancer Treat Rev 2010;36:1-7. [Google Scholar] [PubMed]
- 3. Palmerini E, Picci P, Reichardt P, Downey G. Malignancy in giant cell tumor of bone: A review of the literature. Technol Cancer Res Treat 2019;18. [Google Scholar] [PubMed]
- 4. Shi J, Zhao Z, Yan T, Guo W, Yang R, Tang X, et al. Surgical treatment of benign osteolytic lesions in the femoral head and neck: A systematic review. BMC Musculoskelet Disord 2021;16:549. [Google Scholar] [PubMed]
- 5. Wijsbek AE, Vazquez-Garcia BL, Grimer RJ, Carter SR, Abudu AA, Tillman RM, et al. Giant cell tumour of the proximal femur: Is joint-sparing management ever successful? Bone Joint J 2014;96-B:127-31. [Google Scholar] [PubMed]
- 6. Gaston CL, Bhumbra R, Watanuki M, Abudu AT, Carter SR, Jeys LM, et al. Does the addition of cement improve the rate of local recurrence after curettage of giant cell tumours in bone? J Bone Joint Surg Br 2011;93:1665-9. [Google Scholar] [PubMed]
- 7. Luengo-Alonso G, Mellado-Romero M, Shemesh S, Ramos-Pascua L, Pretell-Mazzini J. Denosumab treatment for giant-cell tumor of bone: A systematic review of the literature. Arch Orthop Trauma Surg 2019;139:1339-49. [Google Scholar] [PubMed]
- 8. Kivioja AH, Blomqvist C, Hietaniemi K, Trovik C, Walloe A, Bauer HC, et al. Cement is recommended in intralesional surgery of giant cell tumors: A scandinavian sarcoma group study of 294 patients followed for a median time of 5 years. Acta Orthop 2008;79:86-93. [Google Scholar] [PubMed]
- 9. Sakayama K, Yamamoto H, Sugawara Y, Kidani T, Miyawaki J, Fujibuchi T, et al. Diagnostic and therapeutic problems of giant cell tumor in the proximal femur. Arch Orthop Trauma Surg 2007;127:867-72. [Google Scholar] [PubMed]
- 10. Turcotte RE, Wunder JS, Isler MH, Bell RS, Schachar N, Masri BA, et al. Giant cell tumor of long bone: A Canadian sarcoma group study. Clin Orthop Relat Res 2002;397:248-58. [Google Scholar] [PubMed]
- 11. Lausten GS, Jensen PK, Schiodt T, Lund B. Local recurrences in giant cell tumour of bone. Long-term follow up of 31 cases. Int Orthop 1996;20:172-6. [Google Scholar] [PubMed]
- 12. Borkowska AM, Szumera-Ciećkiewicz A, Szostakowski B, Pieńkowski A, Rutkowski PL. Denosumab in giant cell tumor of bone: Multidisciplinary medical management based on pathophysiological mechanisms and real-world evidence. Cancers (Basel) 2022;14:2290. [Google Scholar] [PubMed]
- 13. Errani C, Tsukamoto S, Leone G, Righi A, Akahane M, Tanaka Y, et al. Denosumab may increase the risk of local recurrence in patients with giant-cell tumor of bone treated with curettage. J Bone Joint Surg Am 2018;100:496-504. [Google Scholar] [PubMed]
- 14. Perrin DL, Visgauss JD, Wilson DA, Griffin AM, Abdul Razak AR, Ferguson PC, et al. The role of denosumab in joint preservation for patients with giant cell tumour of bone. Bone Joint J 2021;103-B:184-91. [Google Scholar] [PubMed]
- 15. Yuan Y, Liu Q, Liu Y, Wu Z, Zhong W, He H, et al. Comparative analysis of two surgical treatment options for giant cell tumor of the proximal femur: Extended curettage and segmental resection. Front Oncol 2021;11:771863. [Google Scholar] [PubMed]
- 16. Lin F, Hu Y, Zhao L, Zhang H, Yu X, Wang Z, et al. The epidemiological and clinical features of primary giant cell tumor around the knee: A report from the multicenter retrospective study in china. J Bone Oncol 2016;5:38-42. [Google Scholar] [PubMed]
- 17. Errani C, Tsukamoto S, Ciani G, Donati DM. Present day controversies and consensus in curettage for giant cell tumor of bone. J Clin Orthop Trauma 2019;10:1015-20. [Google Scholar] [PubMed]
- 18. Campanacci M, Giunti A, Olmi R. Metaphyseal and diaphyseal localization of giant cell tumors. Chir Organi Mov 1975;62:29-34. [Google Scholar] [PubMed]
- 19. Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res 1993;286:241-6. [Google Scholar] [PubMed]








