Dr. Arvind J Vatkar, Department of Orthoapedics, MGM Medical College, Nerul, Navi Mumbai, Maharashtra, India. E-mail: vatkararvind@gmail.com
Abstract Pseudomeningocele, a post-operative or post-traumatic cerebrospinal fluid (CSF) collection from a dural defect, is an underrecognized complication following spinal or cranial surgery. Incidence varies from 0.07 to 2% in lumbar and up to 23% in posterior fossa procedures. While many resolve spontaneously, symptomatic cases can cause headaches, radiculopathy, or infection, necessitating stepwise management. Diagnosis relies on magnetic resonance imaging findings of fluid continuity with the subarachnoid space. Asymptomatic, small pseudomeningoceles are managed conservatively through bed rest, abdominal binder, analgesics, and serial imaging. Persistent or enlarging lesions require CSF diversion (lumbar drainage), epidural blood patch, or aspiration. Surgery is indicated for refractory cases or complications, emphasizing watertight dural repair using non-absorbable sutures, duraplasty with autologous fascia or synthetic graft, and myofascial flap reinforcement. This structured, evidence-based algorithm integrates conservative, interventional, and surgical strategies to guide clinicians in effectively managing postoperative and post-traumatic pseudomeningoceles.
Keywords: Pseudomeningocele, dural tear, lumbar.
Postoperative and post-traumatic pseudomeningocele is not an uncommon complication following spinal procedures, with reported incidences varying significantly across different studies. The incidence of post-operative pseudomeningocele after lumbar spine surgery ranges from 0.07% to 2% [1]. With some studies reporting rates as high as 13% when including all cerebrospinal fluid (CSF)-related complications. In posterior fossa surgeries, the incidence can reach 4–23% [2]. Despite these relatively significant rates, most cases remain asymptomatic and underreported, as surgeons may be reluctant to document this complication and many resolve spontaneously. There exists a notable paucity of literature regarding standardized management approaches, with most evidence limited to case reports and small retrospective series. At present, no established algorithm or guideline exists for the systematic management of post-traumatic or postoperative pseudomeningocele, leaving clinicians to rely on individualized, symptom-driven approaches extrapolated from limited case experiences and expert opinion rather than evidence-based protocols.
Here is a clinically relevant algorithm for managing pseudomeningocele after spinal surgery, synthesised from current literature and expert practice [3].
Step 1: Diagnosis
Confirm pseudomeningocele with magnetic resonance imaging/computed tomography (MRI/CT) and correlate clinically [4].
Confirmation of Pseudomeningocele: Investigations and Clinical Examination
Clinical examination
Pseudomeningocele is often identified as a fluctuant, palpable subcutaneous lump at the surgery or trauma site (Fig. 1). Key clinical findings include headaches caused by collection palpation, back discomfort, radicular complaints, and, on occasion, neurological abnormalities. The swelling may show positive transillumination and produce symptoms when compressed, indicating CSF hypertension [5].

Figure 1: Globular swelling in pseudomeningoceles in lumbar region.
Imaging investigations
Imaging Investigations: MRI remains the gold standard, with distinctive low signal intensity on T1-weighted pictures and high signal intensity on T2-weighted sequences, which are identical to CSF elsewhere. Direct visualization of connectivity with the subarachnoid region as a fluid tract or jet artefact on T2 sequences is one of the most critical diagnostic findings (Fig. 2). CT scans show hypoattenuating collection with CSF-equivalent attenuation and negligible peripheral enhancement [6].

Figure 2: Magnetic resonance imaging films of the patient showing pseudomeningocele post-surgery – at L45 level.
Step 2: Assess symptoms and size
Asymptomatic and small: No neurological deficit, mild or no pain [7].
Symptomatic or large: Headache, neurological symptoms, increasing size, infection, or wound issues [8].
Symptomatic pseudomeningoceles are frequently associated with headache due to occasional CSF hypertension from compression or CSF hypotension from persistent leakage, resulting in holocranial, posture-specific pain that worsens upright and relieves reclined. Associated symptoms may include syncope, nausea, photophobia, and visual abnormalities. Neurological symptoms develop when growing collections compress neural tissues, resulting in radicular pain, sensory abnormalities, or motor paralysis; uncommon instances report acute myelopathy, decerebrate stiffness, and brainstem compression with coma [9].
Progressive expansion, sometimes caused by a ball-valve mechanism, indicates that conservative management has failed; pseudomeningoceles larger than 5 cm or with growth lasting more than 1–2 weeks require surgery to avoid a mass effect on the spinal cord, trachea, or esophagus in cervical region [10]. The risk of infection increases with prolonged CSF accumulation, leading to meningitis and chronic wound infection; infection rates in afflicted individuals have reached over 46%. Wound issues include breakdown and CSF fistula development, which hamper wound healing, increase infection risk, and might require multiple revisions [11].
Step 3: Initial management
Asymptomatic and small
Conservative care is the primary choice of treatment for individuals with asymptomatic or slightly symptomatic pseudomeningoceles that do not cause substantial neurological impairments or active CSF leaking. Initial observation along with regular clinical examinations is recommended, since many pseudomeningoceles resolve spontaneously over time, with research indicating that 73.5% of cases recover without intervention [13]. Serial imaging with magnetic resonance imaging (MRI) or CT scan measures lesion size, mass effect, and spontaneous resolution. Clinical evaluations use headaches, photophobia, radicular pain, neurology and wound integrity to guide treatment options [8].
Mild symptoms without compression
Conservative: Analgesics, hydration, and close follow-up [12].
Asymptomatic and small pseudomeningoceles are frequently handled noninvasively, with cautious observation rather than urgent treatment. Pseudomeningoceles should be treated conservatively with analgesics to relieve symptoms, enough hydration to maintain appropriate intravascular volume and CSF production, and attentive clinical follow-up. Patients should be observed weekly for the first month to check symptoms and pseudomeningocele size, then evaluated monthly for the next 3 months. Long-term monitoring may last up to 6 months, with imaging scans performed as clinically necessary to check for resolution or progression. The first care comprises of surveillance with serial imaging, often MRI at regular intervals, to verify that the collection remains stable or resolves itself. During this time, patients are encouraged to lie back in bed to prevent CSF dynamics that might increase the sac and symptom provocation. An abdominal binder offers external support by raising intra-abdominal pressure, which reduces CSF leaking through the dural defect [14].
Pseudomeningoceles should be treated conservatively with analgesics to relieve symptoms, enough hydration to maintain appropriate intravascular volume and CSF production, and attentive clinical follow-up. Patients should be observed weekly for the first month to check symptoms and pseudomeningocele size, then evaluated monthly for the next 3 months. Long-term monitoring may last up to 6 months, with imaging scans performed as clinically necessary to check for resolution or progression.
Step 4: Interventional management
Persistent symptoms OR enlarging pseudomeningocele
- Lumbar subarachnoid drainage: Temporary CSF diversion for 3–4 days [15].
Lumbar subarachnoid drainage is a temporary CSF diversion procedure used to treat symptomatic or growing pseudomeningocele. A lumbar drain diverts CSF externally for 3–4 days, reducing pressure at the dural defect location and facilitating dura repair. Typical daily drainage rates vary between 5 and 10 mL/h; careful monitoring prevents problems such as herniation or infection. This method is frequently used in conjunction with primary dural repair and myofascial flap support in surgically treated situations. Typically, on the third day of the drain, it is clamped for 24 h to check for leakage from the wound site before being removed. The therapy has proven to be helpful in clearing collections and promoting wound healing while reducing the chance of recurrence [16].
- Epidural blood patch: For mild-to-moderate leaks not resolving with conservative care or after drainage attempt [3].
- Percutaneous aspiration: Percutaneous aspiration of the CSF can be used as an adjunctive in select cases [17]. But the risk of infection and CSF fistula remain.
Step 5: Surgical management
Failed non-surgical measures or presence of complications
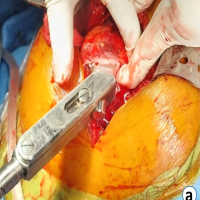
- Direct repair of dural defect with non-absorbable suture [3] (Fig. 3).

Figure 3: Direct open repair of dura site of repair shown with an arrow.
When conservative management fails, and symptoms develop, the optimal surgical treatment for pseudomeningoceles is direct closure of dural lesions with non-absorbable sutures. Non-absorbable sutures, such as 7-0 Prolene, provide a long-lasting, watertight closure that is necessary to prevent persistent CSF leaking [18]. According to studies, interrupted or running locked suturing procedures are equally effective at halting leaks [19]. This repair improves dural integrity, relieves symptoms like headaches and nerve root irritation, and lowers the risk of complications. Although adjuncts such as sealants may be utilized, research indicates that primary suture repair is still the most successful solo approach.
- Duraplasty if defect is large: Use autologous fascia, synthetic graft, or sealant [7].
- Duraplasty is recommended for large dural defects in pseudomeningocele cases where direct repair is inadequate. It entails grafting autologous fascia, synthetic dura augments, or sealants to close the defect. Autologous fascia, such as fascia lata, is recommended due to its biocompatibility and efficacy in restoring dural integrity [3]. Synthetic grafts can also be used depending on the extent of the defect and the patient’s needs. Fibrin glue helps to create a watertight seal across suture lines, lowering the likelihood of postoperative CSF leakage. Surgicel helps by increasing clot formation and acting as a scaffold for fibrin deposition. Together, they work with sutured dural repairs in spine surgery to reduce bleeding, promote tissue adhesion, and aid in healing [20]. Duraplasty reduces CSF leaking, lowers recurrence risk, and promotes tissue repair in big dural holes.
- Excision of a large pseudomeningocele sac is warranted if they are symptomatic and large [3].
- Myofascial flap or tissue reinforcement as needed [7].
- Myofascial flap or tissue reinforcement is an important treatment for healing pseudomeningoceles, particularly in big or recurrent cases. This entails medial advancement of paraspinal musculofascial units to form a layered, “pants-on-vest” waterproof seal over the dural healing site. This approach eliminates dead space, promotes adhesion, and reduces CSF leakage. The pants-on-vest suture method provides a secure closure by overlaying tissue layers, which increases mechanical strength [21]. Myofascial flaps promote healing and reduce recurrence risk, and they are frequently paired with dural repair and lumbar subarachnoid drainage to provide the best results in symptomatic pseudomeningocele patients [7].
Step 6: Special considerations
- Infection: Antibiotics and surgical drainage. The management of pseudomeningocele involves antibiotic coverage tailored to infection risk, commonly intravenous antibiotics guided by culture and sensitivity, typically continued for about 7–14 days depending on clinical response. Acetazolamide is used to decrease CSF production and flow, usually administered at 250–500 mg intravenously every 8 h, with duration individualized based on patient response, often continuing until CSF leakage or intracranial pressure improves. Additional medications may include analgesics for symptom control and, in infectious cases, specific antimicrobials targeting identified pathogens [16].
- Giant pseudomeningocele: Combined surgical and CSF diversion approach [12]. Collections of CSF that are 8 cm in diameter or more are known as giant pseudomeningoceles [22]. They are an uncommon but significant side effect of spinal surgery that requires early intervention since they frequently manifest as pain, headache, nausea, or neurological abnormalities brought on by mass effect or nerve root entrapment. A mix of surgical procedures is used for management, such as lumbar subarachnoid drainage to lower CSF fluid pressure, correction of dural abnormalities, frequently with fascia grafts, and excision of the pseudomeningocele sac [5].
- Follow-up: Serial clinical assessment and imaging to track resolution or recurrence [16] (Table 1).

Table 1: Algorithm providing a structured approach for managing pseudomeningocele after spinal surgery, balancing conservative and interventional steps before surgical repair [15]
References
- 1. Miller PR, Elder FW Jr. Meningeal pseudocysts (meningocele spurius) following laminectomy. Report of ten cases. J Bone Joint Surg Am 1968;50:268-76. [Google Scholar] [PubMed]
- 2. Sastry RA, Walek K, Leary OP, Rex N, Shaaya EA, Poggi JA, et al. Incidence, characteristics, and outcomes of pseudomeningocele and cerebrospinal fluid fistula after posterior fossa surgery. World Neurosurg 2022;164:e1094-102. [Google Scholar] [PubMed]
- 3. Weng YJ, Cheng CC, Li YY, Huang TJ, Hsu RW. Management of giant pseudomeningoceles after spinal surgery. BMC Musculoskelet Disord 2010;11:53. [Google Scholar] [PubMed]
- 4. Gupta R, Narayan S. Post-operative pseudomeningocele after spine surgery: Rare cause of failed back syndrome. Iran J Neurosurg 2016;2:15-8. [Google Scholar] [PubMed]
- 5. Srilomsak P, Okuno K, Sakakibara T, Wang Z, Kasai Y. Giant pseudomeningocele after spinal surgery: A case report. World J Orthop 2012;3:109-13. [Google Scholar] [PubMed]
- 6. Radcliff K, Morrison WB, Kepler C, Moore J, Sidhu GS, Gendelberg D, et al. Distinguishing pseudomeningocele, epidural hematoma, and postoperative infection on postoperative MRI. Clin Spine Surg 2016;29:E471-4. [Google Scholar] [PubMed]
- 7. Oktay K, Güzel E, Akbaba M, Baykara O, Er U, Güzel A. Surgical treatment of iatrogenic pseudomeningoceles. J Turk Spinal Surg 2023;34:71-5. [Google Scholar] [PubMed]
- 8. Kavishwar RA, Shetty AP, Subramanian B, Rajasekaran S. Symptomatic postsurgical lumbar pseudomeningocele treated by ultrasound-guided epidural blood patch application. Asian J Neurosurg 2021;16:827-9. [Google Scholar] [PubMed]
- 9. Sanabria Sanchinel AA, Lin Y, Rodríguez Rubio D. Pseudomeningocele: Headache, apnoea, and syncope. Neurologia 2021;36:654-6. [Google Scholar] [PubMed]
- 10. Rahimizadeh A, Soufiani H, Rahimizadeh S. Remote cervical pseudomeningocele following anterior cervical corpectomy and fusion: Report of a case and review of the literature. Int J Spine Surg 2016;10:36. [Google Scholar] [PubMed]
- 11. Wang J, Yang JB, Wang XL, Ding WL. Pseudomeningocele following posterior cranial fossa surgery significantly increases the risk of intracranial infection: A 10-year retrospective analysis. Surg Infect (Larchmt) 2024;25:598-605. [Google Scholar] [PubMed]
- 12. Pascual-Gallego M, Zimman H, Gil A, López-Ibor L. Pseudomeningocele after traumatic nerve root avulsion. A novel technique to close the fistula. Interv Neuroradiol 2013;19:496-9. [Google Scholar] [PubMed]
- 13. Dustur S, Parenrengi MA, Suryaningtyas W. Management of pseudomeningocele following posterior fossa tumor surgery with absence of hydrocephalus: A case report. Int J Surg Case Rep 2022;98:107552. [Google Scholar] [PubMed]
- 14. Julia PE, Nazirah H. Spinal pseudomeningocoele: A diagnostic dilemma. Spinal Cord 2007;45:804-5. [Google Scholar] [PubMed]
- 15. Sandwell S, Walter K, Westesson PL. Pseudomeningocele aspiration and blood patch effectively treats positional headache associated with postoperative lumbosacral pseudomeningocele. Spine (Phila Pa 1976) 2017;42:1139-44. [Google Scholar] [PubMed]
- 16. Bradaschia L, Lacatena F, Vincitorio F, Titolo P, Battiston B, Garbossa D, et al. Management of post-traumatic pseudomeningocele as consequence of root nerve avulsion: Case report and review of the literature. Neurol Int 2024;16:1742-9. [Google Scholar] [PubMed]
- 17. Chang AH, Seroussi R, Singh V. Symptomatic lumbar pseudomeningocele from post-operative dural leak treated with autologous platelet-rich plasma epidural patch: A case study. Interv Pain Med 2022;1:100093. [Google Scholar] [PubMed]
- 18. Al Jammal OM, Wali AR, Lewis CS, Zaldana MV, Suliman AS, Pham MH. Management of giant sacral pseudomeningocele in revision spine surgery. Int J Spine Surg 2020;14:778-84. [Google Scholar] [PubMed]
- 19. Choi EH, Chan AY, Brown NJ, Lien BV, Sahyouni R, Chan AK, et al. Effectiveness of repair techniques for spinal dural tears: A systematic review. World Neurosurg 2021;149:140-7. [Google Scholar] [PubMed]
- 20. Epstein NE. Hemostasis and other benefits of fibrin sealants/glues in spine surgery beyond cerebrospinal fluid leak repairs. Surg Neurol Int 2014;5 Suppl 7:S304-14. [Google Scholar] [PubMed]
- 21. Misra SN, Morgan HW, Sedler R. Lumbar myofascial flap for pseudomeningocele repair. Neurosurg Focus 2003;15:E13. [Google Scholar] [PubMed]
- 22. Rahimizadeh A, Mohsenikabir N, Asgari N. Iatrogenic lumbar giant pseudomeningocele: A report of two cases. Surg Neurol Int 2019;10:213. [Google Scholar] [PubMed]