In elderly patients with osteoarthritis, simple inflammatory markers that are already part of routine practice appear to move in tandem with pain, functional limitation, and radiographic severity when interpreted in context, rather than in isolation. Their value lies less in diagnosis and more in reflecting the underlying disease burden and inflammatory profile.
Dr. Preeti Nigotia, Department of General Medicine, Shrimant Rajmata Vijayaraje Scindia Medical College, Shivpuri, Madhya Pradesh, India. E-mail: preeti9560@gmail.com
Introduction: Osteoarthritis (OA) remains one of the most frequent causes of chronic pain and disability in older adults. Although traditionally described as a degenerative condition, clinical experience increasingly suggests that inflammatory activity may modulate symptom severity and structural damage. This study was undertaken to examine how routinely measured inflammatory markers relate to clinical status and radiological severity in elderly patients with OA.
Material and Methods: A hospital-based cross-sectional study was conducted among 134 patients aged 60 years or older with clinically and radiologically confirmed OA of the knee and/or hip. Pain severity was assessed using the visual analog scale, while functional impairment was evaluated with the Western Ontario and McMaster Universities OA Index. Radiographic severity was graded according to the Kellgren–Lawrence (K–L) classification. Laboratory assessment included measurement of erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). Statistical analyses were performed to explore associations between inflammatory markers and clinical as well as radiological parameters.
Results: The study cohort was predominantly female, and knee involvement was more frequent than hip disease. Most participants fell within the mild to moderate range of radiological severity. Both ESR and CRP levels increased progressively with higher K–L grades. Differences in inflammatory marker levels across radiological grades were statistically significant. Correlation analysis demonstrated positive associations between inflammatory markers and pain scores, functional limitation, and radiographic severity, indicating that higher inflammatory activity was accompanied by worse clinical and structural disease.
Conclusion: In elderly patients with OA, ESR and CRP show meaningful correlations with pain intensity, functional impairment, and radiological grading. These findings support the presence of a low-grade systemic inflammatory component that parallels disease severity and may influence clinical expression.
Keywords: Osteoarthritis, elderly, inflammatory markers, erythrocyte sedimentation rate, C-reactive protein.
Osteoarthritis (OA) dominates the clinical landscape of musculoskeletal disease in older adults, not because it is dramatic, but because it is persistent, cumulative, and disabling. With increasing age, symptomatic OA becomes almost expected rather than exceptional, and its contribution to chronic pain, loss of mobility, and dependency is substantial [1]. While cartilage degeneration remains a defining structural feature, limiting OA to a purely mechanical or “wear-related” process no longer fits with what is observed clinically or biologically. Present understanding frames OA as a disorder of the entire joint organ, involving cartilage, subchondral bone, synovium, ligaments, and periarticular tissues. These components do not fail in isolation; instead, mechanical stress, biochemical signaling, and altered joint mechanics interact over time, producing variable symptom patterns and structural progression [2]. This broader view has drawn attention to inflammatory activity, not as a dominant driver comparable to inflammatory arthritides, but as a modifying factor that may amplify pain and influence disease trajectory. Low-grade inflammation has emerged as a recurring finding in OA, particularly in older individuals. Synovial thickening, effusion, and increased local production of inflammatory mediators have been linked to symptom severity and, in some studies, to radiographic progression [2,3]. Whether systemic inflammation mirrors these intra-articular changes remains less clear. Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), though non-specific, are attractive in this context because they are inexpensive, widely available, and routinely measured. However, their relevance in OA has been inconsistently reported. Some investigations document mild but meaningful elevations associated with pain or radiographic grade, while others find minimal or absent associations [3,4]. Syntheses of the literature suggest that both local inflammatory features on imaging and certain systemic markers show associations with pain and functional impairment, but these relationships are neither uniform nor strong across studies [4,5]. Differences in age distribution, disease stage, joint involvement, and analytical methods likely contribute to this variability. More recent work examining CRP isoforms and emerging inflammatory biomarkers has raised the possibility that conventional assays may underestimate OA-related inflammatory activity, although such approaches are not yet applicable to routine clinical practice and remain insufficiently validated in elderly populations [6]. In the setting of an ageing population and heterogeneous disease expression, it remains clinically relevant to ask a pragmatic question: do simple inflammatory markers such as ESR and CRP convey meaningful information about disease burden when interpreted alongside standardized clinical assessment and radiographic grading? The present study was designed to explore this question by examining the relationship between ESR, CRP, symptom severity, functional status, and radiological stage in an elderly cohort with OA.
Study design and setting
This hospital-based, cross-sectional observational study was conducted at a tertiary care teaching hospital. Participants were recruited from both outpatient and inpatient services to capture a broad clinical spectrum of elderly patients with OA.
Ethical considerations
Ethical clearance was obtained from the Institutional Ethics Committee before initiation of the study. All study procedures were carried out in accordance with the Declaration of Helsinki. Written informed consent was obtained from each participant after explaining the objectives and procedures of the study, and confidentiality was maintained throughout the study period (2024).
Study population
Elderly patients diagnosed with OA were consecutively enrolled to reduce selection bias. Recruitment included patients presenting for routine evaluation as well as those admitted for symptom management.
Inclusion criteria
Patients aged 60 years and above with a clinical and radiological diagnosis of OA were included. Diagnosis was established according to the American College of Rheumatology criteria. Only individuals with knee and/or hip involvement were considered eligible. Participation was limited to patients who provided written informed consent.
Exclusion criteria
Patients with inflammatory arthropathies such as rheumatoid arthritis, gout, or seronegative spondyloarthropathies were excluded. To avoid confounding effects on inflammatory markers, individuals with acute or chronic infections, malignancy, or autoimmune disorders were not included. Additional exclusions comprised recent trauma or surgical intervention within the preceding 3 months, present use of systemic corticosteroids, immunosuppressive agents, or disease-modifying antirheumatic drugs, and the presence of chronic liver disease, chronic kidney disease, or hematological disorders known to influence inflammatory parameters.
Sample size estimation
Sample size calculation was based on previously reported moderate correlations between inflammatory markers and clinical severity in OA [7]. Assuming a correlation coefficient of 0.3, a 95% confidence level, and 80% power, the minimum required sample size was estimated to be 85 participants. To account for exclusions and incomplete data, a total of 134 patients were ultimately included.
Clinical assessment
Demographic and clinical data were recorded using a structured pro forma, including age, sex, duration of symptoms, pattern of joint involvement, comorbidities, and medication history. Pain severity was assessed using the Visual Analog Scale (VAS) [8]. Functional status was evaluated with the Western Ontario and McMaster Universities OA Index (WOMAC), covering pain, stiffness, and physical function domains [9]. Radiological severity was graded using the Kellgren–Lawrence (K–L) grading system [10] based on standard anteroposterior radiographs of the affected joints.
Laboratory investigations
Venous blood samples were obtained under aseptic precautions after an overnight fast. ESR was measured using the Westergren method, and CRP levels were estimated by a high-sensitivity immunoturbidimetric assay. ESR and CRP were selected because they are routinely available, cost-effective, and commonly used indicators of systemic inflammatory activity. Their inclusion was intended to reflect real-world clinical practice, particularly in elderly patients where low-grade inflammation has been increasingly linked to symptom severity and structural disease burden. Advanced cytokine assays were not pursued due to limited feasibility and to maintain the applicability of findings to routine tertiary care settings. A complete blood count, including total and differential leukocyte counts, was performed using an automated hematology analyzer. All laboratory analyses were carried out in the central clinical laboratory following standardized operating procedures, with internal quality control measures in place.
Outcome measures
The primary outcome was the relationship between inflammatory markers (ESR and CRP) and both clinical and radiological severity of OA. Secondary outcomes included variations in inflammatory marker levels across different K–L grades and their associations with pain intensity and functional impairment.
Statistical analysis
Data were entered into Microsoft Excel and analyzed using the Statistical Package for the Social Sciences version 26. Continuous variables were expressed as mean ± standard deviation, while categorical variables were summarized as frequencies and percentages. Correlations between inflammatory markers and clinical or radiological parameters were assessed using Pearson’s correlation coefficient. Differences in inflammatory marker levels across K–L grades were evaluated using one-way analysis of variance. A P < 0.05 was considered statistically significant.
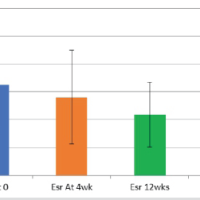
The study cohort consisted of elderly individuals with OA, with women forming a larger proportion of the sample. Participants were distributed across the higher age ranges, reflecting the typical age profile of symptomatic disease. Knee involvement emerged as the most common presentation, either in isolation or in combination with hip involvement. At the time of assessment, most patients described pain and functional difficulty that fell within a moderate range rather than at the extremes. The baseline demographic and clinical profile of the participants is outlined in Table 1. Radiographic assessment showed that OA severity clustered predominantly within the intermediate K–L grades. Mild to moderate radiological disease accounted for the majority of cases, while advanced changes were confined to a smaller fraction of the cohort. This uneven distribution suggests that most patients sought care before reaching end-stage structural damage. The detailed distribution of radiological grades is presented in Table 2. Both inflammatory markers demonstrated a clear upward trend with increasing radiographic severity. Mean ESR and CRP values rose progressively across successive K–L grades, with the highest levels observed in patients with advanced radiological disease. Differences in inflammatory marker levels across radiological grades reached statistical significance, indicating that these changes were unlikely to be due to random variation alone. The association between inflammatory markers and radiological severity is shown in Table 3. Correlation analysis further supported a relationship between systemic inflammation and clinical disease burden. Higher ESR and CRP values were associated with greater pain intensity on the VAS, higher WOMAC scores reflecting worse functional status, and increasing radiological severity. Although the strength of these correlations was moderate, the direction of association was consistent across clinical and structural parameters, suggesting that inflammatory activity parallels both symptom expression and joint damage. These findings are summarized in Table 4. Additional exploratory analyses examined whether inflammatory marker levels varied according to baseline demographic factors. Neither ESR nor CRP differed significantly by sex or by pattern of joint involvement (knee, hip, or combined), and age showed only weak, non-significant correlations with both markers (P > 0.05). Taken together, these observations indicate that the elevations in ESR and CRP observed in this cohort were more closely linked to OA severity than to demographic characteristics alone.
This study explored how routinely measured systemic inflammatory markers relate to both symptoms and structural severity in elderly patients with OA. The pattern that emerged was not subtle: ESR and CRP increased alongside higher K–L grades and tracked with greater pain and functional limitation. While these markers are non-specific, their consistent association with radiographic and clinical indices in this cohort supports the view that low-grade inflammation accompanies, and possibly amplifies, disease expression in OA. This interpretation fits with recent population-level observations showing that synovitis and structural progression influence each other over time, rather than occurring as isolated phenomena [11]. The correlations observed between inflammatory markers and symptom burden are in line with earlier systematic reviews and longitudinal cohorts that report weak to moderate relationships between ESR or CRP and pain, function, or imaging-defined synovitis in knee OA. Importantly, those studies also highlight considerable heterogeneity, which mirrors the variability seen in everyday clinical practice. Differences in age, joint involvement, disease stage, and analytic methods likely explain why systemic markers appear informative in some populations but less so in others [4]. In this context, our findings suggest that, within an elderly cohort, conventional inflammatory markers still capture a clinically relevant signal when interpreted against standardized symptom and radiographic measures. Imaging-based studies provide additional context for these findings. Synovitis has been linked particularly to persistent or background pain rather than activity-related pain alone, and to specific pain phenotypes that are not explained purely by mechanical load. This may explain why ESR and CRP in our cohort showed associations with both pain intensity and WOMAC-derived functional impairment. Systemic inflammatory activity may, in many patients, reflect ongoing intra-articular inflammatory processes that are not fully appreciated on plain radiographs. These observations reinforce the idea that clinical assessment, imaging, and laboratory data together offer a more nuanced understanding of OA-related pain mechanisms than any single measure alone [12]. At the same time, the limitations of ESR and conventional CRP must be acknowledged. These assays reflect generalized systemic inflammation and are unlikely to capture localized or pathway-specific inflammatory activity within the joint. Recent biomarker research has therefore shifted toward more refined measures, such as CRP metabolites and monomeric CRP, which appear to better identify an inflammatory OA phenotype and may carry greater prognostic value in selected cohorts. The moderate strength of associations observed with standard ESR and CRP in our study may reflect this lack of specificity rather than the absence of inflammatory involvement [13,14]. Variation across published studies also stems from methodological differences. Cross-sectional designs, such as ours, capture a single time point and cannot address whether elevated inflammatory markers precede structural worsening or simply rise in parallel with established disease. Differences in radiographic scoring systems, pain phenotyping, laboratory assay sensitivity, and comorbidity profiles further complicate comparisons. While our data demonstrate meaningful clinico-laboratory relationships, longitudinal studies incorporating sensitive biomarkers and imaging-defined synovitis are needed to clarify whether systemic inflammation predicts progression or treatment response in elderly OA populations [15]. From a clinical standpoint, the findings support a pragmatic, rather than deterministic, role for ESR and CRP in OA. When elevations occur alongside worsening pain or clinical suspicion of synovitis, these markers may justify closer evaluation or influence anti-inflammatory management strategies. However, they should not be used in isolation for disease staging or prognostication. Their greatest utility appears to lie in helping identify an inflammatory phenotype within an otherwise heterogeneous OA population, a role that may be refined further as novel biomarkers and standardized imaging protocols become more accessible [4,13]. Several limitations warrant consideration. The cross-sectional nature of the study precludes inference about causality or temporal relationships. Despite careful exclusion of conditions known to influence inflammatory markers, residual confounding from age-related comorbidities and subclinical inflammatory states cannot be entirely excluded. Inflammatory markers were measured contemporaneously with clinical assessment but were not timed to acute symptom flares, suggesting that the observed associations reflect baseline inflammatory burden rather than transient fluctuations. Strengths of the study include a well-defined elderly cohort, standardized radiographic grading, and parallel clinical and laboratory evaluation. Future work should focus on longitudinal follow-up, incorporation of imaging-defined synovitis using ultrasound or contrast-enhanced magnetic resonance imaging, and assessment of emerging biomarkers such as C-reactive protein metabolite, Monomeric C reactive protein, and cytokine panels to better delineate inflammatory phenotypes and their clinical relevance in OA [11,13,14].
In this elderly cohort with OA, systemic inflammatory markers were not merely incidental findings but showed consistent associations with both symptom burden and radiographic severity. Rising ESR and CRP levels accompanied higher pain scores, greater functional impairment, and more advanced K–L grades. Although these markers are non-specific, their parallel increase with clinical and structural disease measures supports the presence of a low-grade inflammatory component that shapes how OA manifests and progresses in older patients. Within this context, ESR and CRP appear to have value as adjunctive measures, complementing clinical evaluation and radiological grading rather than replacing them. Integrating clinico-laboratory information may allow a more nuanced assessment of disease burden and support stratified, patient-centered management in elderly individuals with OA.
OA in older adults often extends beyond mechanical joint degeneration and is frequently accompanied by low-grade systemic inflammation. Commonly available tests such as ESR and CRP, when interpreted alongside pain assessment and radiographic findings, can offer additional insight into disease severity. Elevated values may signal a more symptomatic or inflammatory disease profile, helping clinicians identify patients who may benefit from closer monitoring or tailored therapeutic approaches.
References
- 1. Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage 2013;21:1145-53. [Google Scholar] [PubMed]
- 2. Knights AJ, Redding SJ, Maerz T. Inflammation in osteoarthritis: The latest progress and ongoing challenges. Curr Opin Rheumatol 2023;35:128-34. [Google Scholar] [PubMed]
- 3. Hanada M, Takahashi M, Furuhashi H, Koyama H, Matsuyama Y. Elevated erythrocyte sedimentation rate and high-sensitivity C-reactive protein in osteoarthritis of the knee: Relationship with clinical findings and radiographic severity. Ann Clin Biochem 2016;53:548-53. [Google Scholar] [PubMed]
- 4. Dainese P, Wyngaert KV, De Mits S, Wittoek R, Van Ginckel A, Calders P. Association between knee inflammation and knee pain in patients with knee osteoarthritis: A systematic review. Osteoarthritis Cartilage 2022;30:516-34. [Google Scholar] [PubMed]
- 5. Van Berkel AC, Van Spil WE, Schiphof D, Runhaar J, Van Ochten JM, Bindels PJ, et al. Associations between biomarkers of matrix metabolism and inflammation with pain and fatigue in participants suspected of early hip and or knee osteoarthritis: Data from the CHECK study. Osteoarthritis Cartilage 2022;30:1640-6. [Google Scholar] [PubMed]
- 6. Liang Y, Xu K, Liu W, Liu X, Yuan P, Xu P, et al. Monomeric C-reactive protein level is associated with osteoarthritis. Exp Ther Med 2022;23:277. [Google Scholar] [PubMed]
- 7. Karaman N, Ulusoy A, Karaman M. Is there a relationship between blood inflammation markers and the severity of knee osteoarthritis? Turk J Phys Med Rehabil 2024;71:102-8. [Google Scholar] [PubMed]
- 8. Koo M, Yang SW. Visual analogue scale. Encyclopedia 2025;5:190. [Google Scholar] [PubMed]
- 9. Rooba, Shetty S, Samuel AJ. Kannada translation and validation of Western Ontario and McMaster universities osteoarthritis index (WOMAC) in knee osteoarthritis: Kannada version of the WOMAC (K-WOMAC). Indian J Orthop 2025;59:1896-910. [Google Scholar] [PubMed]
- 10. Kohn MD, Sassoon AA, Fernando ND. Classifications in brief: Kellgren-Lawrence classification of osteoarthritis. Clin Orthop Relat Res 2016;474:1886-93. [Google Scholar] [PubMed]
- 11. Jiang T, Weng Q, Liu K, He H, Zhang Y, Zhang W, et al. Bidirectional association identified between synovitis and knee and hand osteoarthritis: A general population-based study. Lancet Reg Health West Pac 2024;50:101169. [Google Scholar] [PubMed]
- 12. Philpott HT, Birmingham TB, Pinto R, Primeau CA, Arsenault D, Lanting BA, et al. Synovitis is associated with constant pain in knee osteoarthritis: A cross-sectional study of OMERACT knee ultrasound scores. J Rheumatol 2022;49:89-97. [Google Scholar] [PubMed]
- 13. Bay-Jensen AC, Bihlet A, Byrjalsen I, Andersen JR, Riis BJ, Christiansen C, et al. Serum C-reactive protein metabolite (CRPM) is associated with incidence of contralateral knee osteoarthritis. Sci Rep 2021;11:6583. [Google Scholar] [PubMed]
- 14. Ruiz-Fernández C, Gonzalez-Rodríguez M, Francisco V, Rajab IM, Gómez R, Conde J, et al. Monomeric C reactive protein (mCRP) regulates inflammatory responses in human and mouse chondrocytes. Lab Invest 2021;101:1550-60. [Google Scholar] [PubMed]
- 15. Lawrence A, Boesel J, Martinez Aguilar R, Gryczewski D, Moni AS. A review and meta-analysis of biomarkers in early-stage osteoarthritis. Orthop Surg 2025;17:1913-23. [Google Scholar] [PubMed]