3D-printed titanium mesh implants offer a feasible option for addressing critical-size bone defects in distal tibial open fractures, promoting osteointegration and enabling early weight-bearing.
Dr. Avinash Kachare, Department Of Orthopedics, LTMMC and GH, Sion, Mumbai, Maharashtra, India. E-mail: theprotonium@protonmail.com
Introduction: Critical-size bone defects in distal tibial open wounds pose a formidable challenge, requiring interventions that can address osseous reconstruction with less number of surgeries. Current treatment modalities may fall short in achieving optimal outcomes, with respect to early weight bearing due to the inability of the graft to sustain weight, graft-related infections, non-union in large defects, donor site morbidity, and non-availability of bone grafts due to earlier harvest. This case report explores the potential application of a 3D-printed mesh implant to this complex clinical scenario.
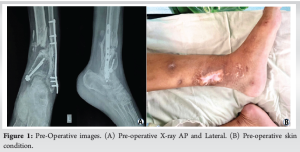
Case Report: A 38-year-old male, post-road traffic accident, presented with an inability to walk due to fractures of the medial malleolus and distal tibia-fibula with a grade 3b open wound. Initial treatment was done with an external fixator and fibula plating, with the fixator removed after 5 months. The fracture showed atrophic non-union and a 2.5 cm limb shortening at the end of 5 months. Preferring thick flap incisions, the patient operated with a 3D-printed titanium mesh implant with a plate construct. Post-surgery, we followed a non-weight-bearing regime for 1.5 months, progressing to full weight-bearing by 3 months. At 1.5 years, CT scans confirmed good bone integration and ambulation restoration.
Conclusion: The use of 3D-printed mesh implants may be a viable option for managing critical-size bone defects in distal tibial open wounds. Porotic nature of mesh implant facilitates bone ingrowth in large gaps.
Keywords: 3D-printing, mesh implant, non-union.
Bone defects pose a significant challenge in orthopedic surgery, particularly when the defect size surpasses the body’s inherent healing capacity. While bone defects <5 cm are typically managed with direct bone grafting, larger defects often lead to non-union due to insufficient regenerative potential [1]. For such critical-sized defects, current treatment modalities include stabilization with bone grafting, bone transport surgery, and vascularized fibula grafts. However, these approaches are not without their drawbacks, such as graft site morbidity and procedural bulkiness [2,3]. In addition, graft resorption remains a notable challenge in the context of large bone defects. In the management of femoral defects ranging from 6 to 15 cm, favorable outcomes are often achieved due to the substantial muscle mass in the area. Conversely, smaller tibial defects, particularly those exceeding 1–2 cm in size and involving more than 50% of the cortical circumference, tend to result in poorer outcomes [4,5]. Critical-size defects, defined as those greater than 1 cm in length and encompassing more than 50% of the cortical diameter, consistently present union challenges even after bone grafting [6]. Tibial defects of 2.5 cm or more are especially susceptible to non-union, with the risk exacerbated by infection and soft tissue damage [7]. Traditional gold standard treatments for large bone defects in weight-bearing bones generally rely on “bone-bone” fusion techniques, such as bone grafting and bone transport. Nonetheless, these methods are associated with several limitations, including the need for revision surgeries, graft site morbidity, and the bulkiness of the grafts, which can be inconvenient for patients [8,9]. In this context, we present a case report of a patient with distal tibia non-union successfully treated using a 3D-printed titanium mesh implant. This innovative approach offers a promising alternative to conventional methods, potentially addressing some of the inherent limitations and improving patient outcomes.
A 38-year-old male presented to OPD with an inability to walk, non-weight-bearing. According to the history, the patient had a road traffic accident (RTA) 1 year prior, which resulted in fractures of the medial malleolus and distal tibia-fibula with a grade 3b open wound. This was managed by an external fixator and fibula plating 1 year prior. Open wound was treated with vacuum-assisted closure dressing. The external fixator was removed at the 5th month post-operatively. Following this patient was kept under immobilization. On X-ray there was an atrophic non-union at the fracture site. Clinically patient had a 2.5 cm shortening on the affected limb (Fig. 1a and b). For treatment, the Ilizarov ring fixator and fibula strut graft option were considered. The patient notably had a version toward any type of external fixator. Due to the need for a minimum incision and early weight bearing; the decision was made to proceed with a titanium mesh implant with plate construct. The patient was informed about the nature of the implant and potential outcomes. The patient accepted the advantages of definitive fixation while being aware of backup plans involving a ring fixator or strut graft if needed.
During surgical planning, a bilateral lower limb CT scan was used to make an anatomically 3d-printed implant by mirroring the normal tibia at the anatomical site. In MIMICS software by Materialise, a CT DICOM was imported, and equivalent cuts were made on the normal tibia by measuring the location of defect from a proximal and distal end of the tibia and normal tibial segment was isolated which was then mirrored. This segment was put up in a lattice structure with mesh stacks in software. To reduce weight of the implant cylindrical cavity of diameter 8 mm was made inside the design. This implant design was supported with a dynamic compressive plate, which was also 3d-printed. Polymer models were printed and fitted with the patient’s printed tibia to check for reproducibility of design in practice (Fig. 2a-d). Pre-operatively, the patient was started with pre-rehabilitation achieving neutral to 20° plantar flexion. During surgery, a thick flap incision was made to minimize complications. After exposure, the fibular plate and medial malleolus screw were removed. Planned cuts were made on the tibia for placing the implant by using Jig. Proper positing of the implant was confirmed under C-arm and the Implant was fixed with corticocancellous screws (Fig. 3a and b). The distal fragment of the distal tibia was noted to be osteoporotic during surgery. A thorough wash was given and wound closure was done in layers. Wound healing was uneventful.


Post-operatively, the patient was followed up under non-weight-bearing for 1.5 months. The patient was kept on partial weight-bearing. At the end of 3rd month, the patient achieved full weight-bearing and restored ambulation, repeat X-ray performed showed the implant in place without any sign of loosening (Fig. 3c). The patient was followed up closely and adequate physiotherapy was continued (Video 1).
At the end of the 1.5 year, a CT scan was performed to check for integration of the implant with bone. CT scan showed two patterns of union; bone bridging through the canal along and bridging callus at the proximal end and some bone growth at the distal end (Fig. 3d) (Video 2).
Current clinical methods to deal with bone losses primarily include bone transport, autograft, and allografts. Bone grafts exhibit important properties of osteoconductive and osteoinductive, but repeated availability can be limited due to multiple number of revision surgeries. They also pose risks, such as donor site morbidity, infection, inadequate integration of graft, devitalization, resorption, and leading to diminished mechanical stability [10,11]. In recent years, implants printed by 3D-technology are gaining popularity in hip and knee arthroplasty. Parameters, such as pore size, density, and topological patterns can be precisely adjusted in newer generation 3D-printing machines, hence implant usability due to better biocomparability has increased [12,13]. In materials used, Titanium (Ti) has the advantage of being light and strong when fabricated, hence it is commonly used [14]. In previous few cases, large cages have been used for bone loss cases in the distal tibia, but such cage was used for total ankle arthrodesis [15]. Varieties of pore diameter and their bone ingrowth capacity have been tested among them 3D-printed implants with pore diameter 700 um has shown more bone ingrowth compared to other sizes of 300 μm, 900 μm, or larger diameter. The concept of bone-to-implant fusion without putting any bone graft is based on the fusion optimum pore size, strut diameter, and porosity of implants [16,17]. We used titanium alloy powder in the DMLS EOS M280 printer to stratify the implant as per specification. The use of a 3D-printed implant with an anatomic design offers another feasible option for addressing critical-size bone defects. This aligns with findings by Teng Zhang et al. who proposed a concept of bone-to-implant fusion without bone grafting [18]. The advantages of this method over the Masquelet technique can be taken as short time for weight bearing, no donor graft site morbidity, and immediate stable construct [19]. Looking at the only complication study involving 3D-printed implants in open wounds found that 10/39 required removal due to non-union, which is comparable to existing methods of fixation [20]. In our case, the Mesh plate construct allowed additional space for bony ingrowth through a canal of mesh implant. The stability of the bone implant construct was achieved partially by intraimplant bone bridging and mesh-plate construct. Based on the findings of our study, this technique may prove highly beneficial in places where there is a shortage of bone grafts, large bone defects, critical-sized bone defects as well as in weight-bearing limbs following bone resection. However, further high-quality randomized control trials are necessary to validate the results.
The low young, modulus of mesh implants serves to minimize the stress shielding around bone implant junction. Enough sturdiness of the implant allowed early weight bearing along with partial bone bridging. The pattern of bone ingrowth was along the mesh as well as inside the canal. This Method of fixation can be considered as one of the sustainable way to treat large bone gaps, instances where large bone grafts are not available may be due to repeated surgeries. Further research and longitudinal studies will be important for the long-term efficacy and reproducibility of this technique.
This case report shows the usability of metal 3D-printing and the importance of surface properties of mesh implants such as porous structures.
References
- 1.GBD 2019 Fracture Collaborators. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990-2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev 2021;2:e580-92. [Google Scholar | PubMed]
- 2.Migliorini F, La Padula G, Torsiello E, Spiezia F, Oliva F, Maffulli N. Strategies for large bone defect reconstruction after trauma, infections or tumour excision: A comprehensive review of the literature. Eur J Med Res 2021;26:118. [Google Scholar | PubMed]
- 3.Quinnan SM, Lawrie C. Optimizing bone defect reconstruction-balanced cable transport with circular external fixation. J Orthop Trauma 2017;31:e347-55. [Google Scholar | PubMed]
- 4.Schemitsch EH. Size matters: Defining critical in bone defect size! J Orthop Trauma 2017;31 Suppl 5:S20-2. [Google Scholar | PubMed]
- 5.Hinsche AF, Giannoudis PV, Matthews SE, Smith RM. Spontaneous healing of large femoral cortical bone defects: Does genetic predisposition play a role? Acta Orthop Belg 2003;69:441-6. [Google Scholar | PubMed]
- 6.Sanders DW, Bhandari M, Guyatt G, Heels-Ansdell D, Schemitsch EH, Swiontkowski M, et al. Critical-sized defect in the tibia: Is it critical? Results from the SPRINT trial. J Orthop Trauma 2014;28:632-5. [Google Scholar | PubMed]
- 7.Haines NM, Lack WD, Seymour RB, Bosse MJ. Defining the lower limit of a “0critical bone defect” in open diaphyseal tibial fractures. J Orthop Trauma 2016;30:e158-63. [Google Scholar | PubMed]
- 8.El-Hadidi TT, Soliman HM, Farouk HA, Radwan MA. Staged bone grafting for the management of segmental long bone defects caused by trauma or infection using induced-membrane technique. Acta Orthop Belg 2018;84:384-96. [Google Scholar | PubMed]
- 9.Misch CM. Complications of autogenous bone grafting. In: Fraum SJ, editor. Dental Implant Complications: Etiology, Prevention and Treatment. United States: Wiley Online Library; 2015. p. 332-61. [Google Scholar | PubMed]
- 10.den Boer FC, Wippermann BW, Blokhuis TJ, Patka P, Bakker FC, Haarman HJ. Healing of segmental bone defects with granular porous hydroxyapatite augmented with recombinant human osteogenic protein-1 or autologous bone marrow. J Orthop Res 2003;21:521-8. [Google Scholar | PubMed]
- 11.Liu G, Zhao L, Zhang W, Cui L, Liu W, Cao Y. Repair of goat tibial defects with bone marrow stromal cells and beta-tricalcium phosphate. J Mater Sci Mater Med 2008;19:2367-76. [Google Scholar | PubMed]
- 12.Barbas A, Bonnet AS, Lipinski P, Pesci R, Dubois G. Development and mechanical characterization of porous titanium bone substitutes. J Mech Behav Biomed Mater 2012;9:34-44. [Google Scholar | PubMed]
- 13.Hanzlik JA, Day JS, Acknowledged Contributors: Ingrowth Retrieval Study Group. Bone ingrowth in well-fixed retrieved porous tantalum implants. J Arthroplasty 2013;28:922-7. [Google Scholar | PubMed]
- 14.Shen H, Brinson LC. A numerical investigation of porous titanium as orthopedic implant material. Mech Mater 2011;43:420-30. [Google Scholar | PubMed]
- 15.Nwankwo EC, Chen F, Nettles DL, Adams SB. Five-year follow-up of distal tibia bone and foot and ankle trauma treated with a 3D-printed titanium cage. Case Rep Orthop 2019;2019:7571013. [Google Scholar | PubMed]
- 16.Feng X, Ma L, Liang H, Liu X, Lei J, Li W, et al. Osteointegration of 3D-printed fully porous polyetheretherketone scaffolds with different pore sizes. ACS Omega 2020;5:26655-66. [Google Scholar | PubMed]
- 17.Taniguchi N, Fujibayashi S, Takemoto M, Sasaki K, Otsuki B, Nakamura T, et al. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: An in vivo experiment. Mater Sci Eng C Mater Biol Appl 2016;59:690-701. [Google Scholar | PubMed]
- 18.Zhang T, Wei Q, Zhou H, Jing Z, Liu X, Zheng Y, et al. Three-dimensional-printed individualized porous implants: A new “implant-bone” interface fusion concept for large bone defect treatment. Bioact Mater 2021;6:3659-70. [Google Scholar | PubMed]
- 19.Wang X, Luo F, Huang K, Xie Z. Induced membrane technique for the treatment of bone defects due to post-traumatic osteomyelitis. Bone Joint Res 2016;5:101-5. [Google Scholar | PubMed]
- 20.Abar B, Kwon N, Allen NB, Lau T, Johnson LG, Gall K, et al. Outcomes of surgical reconstruction using custom 3d-printed porous titanium implants for critical-sized bone defects of the foot and ankle. Foot Ankle Int 2022;43:750-61. [Google Scholar | PubMed]