The integration of in-house 3D printing for surgical guides in orthopedic oncology emphasizes the importance of rigorous quality control measures to ensure accuracy and reliability in patient care.
Dr. Anna Bertoli Borgognoni, Department of Orthopaedic Oncology, Aarhus University Hospital, Palle Juul-Jensens Boulevard 165, 8200 Aarhus N, Denmark. E-mail: annabertoli@clin.au.dk
Introduction: In recent years, numerous hospitals have established in-house three-dimensional (3D) printing centers, enabling health-care facilities to leverage the transformative capabilities of additive manufacturing technology on their premises. With this emerging opportunity arises a necessity to undertake a thorough assessment of the manufactured tools employed in clinical practice. The objectives of this article are to describe the pathway of in-house printing and evaluate the accuracy of 3D-printed specific instruments.
Case Report: A case is reported along with the workflow for creating a patient model and cutting guide. The patient is a 76-year-old Caucasian woman with bone metastasis from a known renal cancer located in the pelvis. The model was used preoperatively, while the guide was used during surgery. Following this, the guide underwent computed tomography (CT) scanning, and a 3D digital model was reconstructed. Two dimensions, labeled A and B, were established. We compared pre-operative measurements, respectively, with measurements from the printed physical guide and from the rescanned post-operative digital model. Finally, A and B were measured on the bone defect on the patient’s post-operative CT. Variation in axis A value between the mean of the first two groups was 0.5 mm and in axis B was 0.7 mm. On the printed physical guide, the mean of axis A was 73.5 mm, and the mean of axis B was 71.8 mm. Variation in A value between the mean of this group and the pre-operative was 1.7 mm and in B value was 0.5 mm.
Conclusion: The workflow used at our hospital was described with an example of how to evaluate the accuracy of in-house 3D printing. Results showed high accuracy of the printing method, a reliable correlation between desired and actual outcomes, and a short lead time.
Keywords: 3D printing, cutting guide, in-house printing.
The integration of 3D printing and computer navigation technology in orthopedic surgery has significantly impacted the way surgical procedures are planned and performed [1,2]. This is especially important in orthopedic oncology, where planning and accurate execution are crucial to obtaining adequate surgical margins. However, as in any new emerging technology, quality control measures need to be implemented to ensure that the output is accurate and reliable, especially with the rise in independent 3D printing centers inside the hospital [3]. In this case report, we want to provide a template to evaluate the geometric accuracy of an in-house 3D-printed cutting guide used during an oncological procedure. The aims of this case study are to describe the pathway of in-house printing and evaluate the accuracy of a printed cutting guide.
This case report was conducted as an Institutional Review Board-approved study.
Patient characteristics
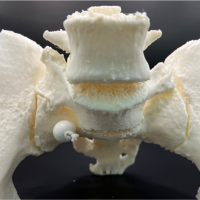
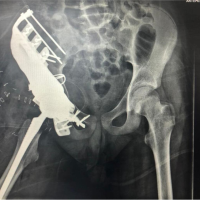
The patient was a 76-year-old Caucasian woman, who presented with pain in the left hip. The CT scan revealed a metastasis from a known renal cancer (clear cell adenocarcinoma), which involved the inferior part of the ilium, the sacroiliac joint, and the sacrum in proximity with the neuroforamina (Fig. 1). The patient was selected for the use of a cutting guide due to the anatomical location of the tumor, which comprises complicated osteotomies and around the pelvis and close relation with the neuroforamina in the sacrum. In December 2022, the patient underwent surgery with resection and reconstruction with a femoral head allograft and two screws. Histology revealed complete tumor removal with negative margins. The patient underwent a post-operative CT (Fig. 2) and was discharged after 14 days. Follow-up was at 2 months with minimal pain and the patient was able to walk without aid inside the house.
3D model and cutting guide: workflow
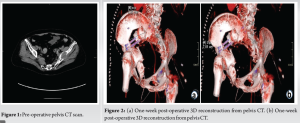
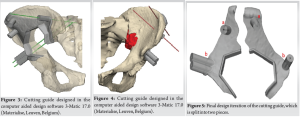
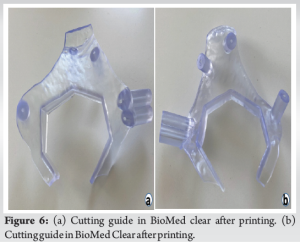
As soon as the case was deemed eligible for a 3D model and cutting guide, a pre-operative CT scan was performed with a CT scanner (Siemens Somatoform Definition Flash, Siemens Healthineers, Erlangen, Germany) with a slice thickness of 1.25 mm. DICOM images were post-processed and a digital 3D model was created by segmentation in Mimics 25.0 (Materialise, Leuven, Belgium) by the engineering team at the innovation department. The tumor was identified by the surgeon in collaboration with an in-house engineer and the desired margin was established. The cutting guide was designed in the computer-aided design software 3-Matic 17.0 (Materialise, Leuven, Belgium), and the first iteration of the guide design was evaluated by the leading surgeon and the in-house engineer (Fig. 3 and 4). The final iteration of the cutting guide was made with cylinders for placement of k-wires: Two k-wires for fixation of the guide to the bone during resection, and 3 k-wires for possible placement of screws. The possible placement for the screws was identified on the digital model with the aim of seeing the intersection with the sacrum. The cutting guide was lastly split into two pieces to enable partial removal while the k-wires for screw placement could remain (Fig. 5). The final design iteration of the cutting guide design was approved by the leading surgeon. The digital model was then exported to an STL file and prepared for printing in preform and printed (Fig. 6) on a Form 3B 3D printer in the biocompatible material BioMed Clear (FormLabs, Somerville, Massachusetts, US). A model of the pelvis and a model of the cutting guide were also produced in polylactic acid on a Sindoh 3DWOX 7X 3D Printer (Sindoh, Seoul, South Korea). These models were for surgery preparation and visualization during surgery (Fig. 7). The lead time for the entire process was 48 h.
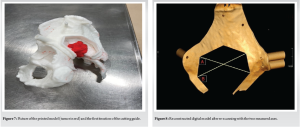
The model was used preoperatively, both by the surgical team for preparation and by the surgeon to explain the details of the upcoming operation to the patient. The cutting guide was sterilized (autoclave) and used in the field during the surgery, and the model was also present in the theater and used as a referral. The guide was utilized as intended. The engineer was present in the operating theatre during the procedure to gather feedback on the design of the cutting guide and to enhance comprehension of anatomical structures and associated challenges with exposure.
Evaluation
Postoperatively, the cutting guide was CT-scanned, and a digital model was recreated. Two diameters (A and B) were defined within the guide (Fig. 8). Three engineers were asked to measure the diameters on the pre-operative first digital 3D model. Three radiologists measured A and B on the post-operative digital model. Three surgeons then measured the same diameters on the physical guide with the help of electronic calipers. Multiple measurements were performed to evaluate the precision of both methods. In addition, one radiologist measured the two diameters of the bone defect on the post-operative CT (Fig. 2). Measurements derived from the pre-operative digital file (engineers) were then compared, respectively, with the printed physical guide (surgeons) and the rescanned post-operative digital model (radiologists).
Small differences were found between the pre-operative digital file and the rescanned post-operative digital model (Table 1). Variation in axis A value between the mean of these two groups was 0.5 mm and in axis B was 0.7 mm. Variation in repeated measurements was 0 in both groups.
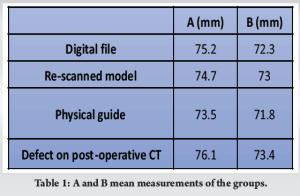
Variation in A value between the mean of the printed physical guide group, and the pre-operative digital file group was 1.7 mm, and in B value was 0.5 mm.
Repeated measurements had a mean variation of 0.4 mm (0-0.9). Finally, the two diameters of the bone defect on the post-operative CT were 76.1 mm for A and 73.4 mm for B.
Workflow
An example is presented of the workflow to produce a 3D printing model and cutting guide, in an in-house printing center, in the context of orthopedic oncology. The printed model proved itself effective in pre-operative planning, where the two leading surgeons had the possibility to discuss details of the operation. The model was also useful to introduce and explain the procedure to the patient step by step, leading to a better understanding of the procedure and improving compliance. Our experience indicates that having at least one engineer present in the operating theater during the procedure is an essential component of the process. It fosters a deeper understanding between the distinct approaches involved in a shared project and facilitates advancements in future endeavors. This has been underlined from the results of other studies, where the engaging collaboration of doctors and engineers was proved to have high effectiveness regarding the successful creation of tools that could be used in actual procedures with high efficacy [4,5]. Another major advantage of in-house production of models and guides is the considerably decreased lead time. Lead time for guide production is difficult to determine from the literature, but, according to Wang et al. study, in which prostheses were printed by electron beam melting technology (ARCAM Q10, Mölndal, Sweden), the manufacturing process of these prostheses entailed a duration of up to 4 weeks [6].
Guide evaluation
As discussed in previous articles there are various methods to evaluate the accuracy of a printed model [3,7-10]. We aimed to extend this concept to our in-house printing operations, focusing particularly on the development of a cutting guide. This emphasis is pertinent as the guide plays a crucial role during the actual surgical procedure. Hedelin et al. pointed out that, if a team wants to evaluate the accuracy of their printing process during the establishment of an in-house printing center, it would be best to do it with an image-based strategy rather than to use calipers [3]. We suggest a method which can be easily applied to every printed guide and is feasible for every hospital which acquires a 3D printing center and wants to demonstrate that the printing is reliable. Two diameters (A and B) were defined within the guide. This decision was taken due to the confirmation of the specific guide and should be adapted for every single case. A difference of 0.57 mm and 0.67 mm, respectively, in axes A and B was found in measurements of the pre-operative and post-operative digital models. This is a remarkable find if we consider the margin of error of the printing, the sterilization process, and the error you introduce when you use a CT scan to reacquire the digital images from the cutting guide postoperatively. Our results obtained from the printed physical guide show a wider range of variability in axis A from the original pre-operative measurements (1.77 mm). Axis B seems to be more accurate (0.53 mm) and similar to what we found in the post-operative digital model. The measurements in this case are obtained with a caliper. This instrument is often a more immediate and affordable tool, but it presents some limitations: It is difficult to standardize the way in which different persons measure the defined axes. This can be seen in the variation between repeated measurements in this group, which is higher than in both digital groups. It is furthermore sometimes challenging or impossible to reach the axes we want to measure due to physical impediments. On the contrary, the internal variation in the digital groups was null, which underlines the higher precision of this method. Of course, this is only an evaluation of the accuracy of the printing process and not of the way in which the guide fits the anatomy of the patient during surgery. Furthermore, we assessed the difference between the desired outcome (a measure of the diameters on the pre-printing digital model) and the actual obtained result on the post-operative CT (Fig. 2). Here a difference was found in diameter A of 0.86 mm and 1.37 mm in diameter B. A previous study with Sawbones specimens have been shown that the mean deviation for their cutting guide ranged from 2.86 mm to 6.54 mm, and they determined that a jig design should have a safety margin of 4.8 mm for standard guides and 8.65 for gusset guides to minimize the risk of cutting into the tumor as a result of human error in guide placements [11]. Other studies should be performed in cadavers and patients to evaluate this specific risk.
In-house printing is a developing reality in many hospitals. This article describes the workflow used at our hospital, and we show an example of how to evaluate the accuracy of the printing. Results showed high accuracy of the printing method and a reliable correlation between desired and actual outcomes in terms of reproducibility, with minimal variation from the pre-operative surgery plan. Lead time is also a crucial factor in producing customized operative tools, we showed that this can be reduced with in-house printing.
This study emphasizes the importance of quality control measures in ensuring the accuracy and reliability of 3D-printed surgical guides, especially with the increasing prevalence of in-house 3D-printing centers within hospitals. The case presentation demonstrates the successful use of a 3D-printed cutting guide in the resection and reconstruction of a metastatic lesion involving complex anatomical structures. The workflow from CT scan to 3D model creation to guide design and printing is outlined, emphasizing the collaborative efforts between surgeons and engineers.
References
- 1.Thadani VN, Riaz MJ, Singh G. The evolution of three-dimensional technology in musculoskeletal oncology. J Clin Orthop Trauma 2018;9:269-74. [Google Scholar | PubMed]
- 2.Wong KC. 3D-printed patient-specific applications in orthopedics. Orthop Res Rev 2016;8:57-66. [Google Scholar | PubMed]
- 3.Hedelin H, Swinkels CS, Laine T, Mack K, Lagerstrand K. Using a 3D printed model as a preoperative tool for pelvic triple osteotomy in children: Proof of concept and evaluation of geometric accuracy. J Am Acad Orthop Surg Glob Res Rev 2019;3:e074. [Google Scholar | PubMed]
- 4.Frizziero L, Santi GM, Leon-Cardenas C, Ferretti P, Sali M, Gianese F, et al. Heat sterilization effects on polymeric, FDM-optimized orthopedic cutting guide for surgical procedures. J Funct Biomater 2021;12:63. [Google Scholar | PubMed]
- 5.Kveller C, Jakobsen AM, Larsen NH, Lindhardt JL, Baad-Hansen T. First experiences of a hospital-based 3D printing facility - an analytical observational study. BMC Health Serv Res 2024;24:28. [Google Scholar | PubMed]
- 6.Wang Y, Min L, Lu M, Zhou Y, Wang J, Zhang Y, et al. The functional outcomes and complications of different reconstruction methods for giant cell tumor of the distal radius: Comparison of osteoarticular allograft and three-dimensional-printed prosthesis. BMC Musculoskelet Disord 2020;21:69. [Google Scholar | PubMed]
- 7.Rengier F, Mehndiratta A, von Tengg-Kobligk H, Zechmann CM, Unterhinninghofen R, Kauczor HU, et al. 3D printing based on imaging data: Review of medical applications. Int J Comput Assist Radiol Surg 2010;5:335-41. [Google Scholar | PubMed]
- 8.Bücking TM, Hill ER, Robertson JL, Maneas E, Plumb AA, Nikitichev DI. From medical imaging data to 3D printed anatomical models. PLoS One 2017;12:e0178540. [Google Scholar | PubMed]
- 9.Wu XB, Wang JQ, Zhao CP, Sun X, Shi Y, Zhang ZA, et al. Printed three-dimensional anatomic templates for virtual preoperative planning before reconstruction of old pelvic injuries: Initial results. Chin Med J (Engl) 2015;128:477-82. [Google Scholar | PubMed]
- 10.Auricchio F, Marconi S. 3D printing: Clinical applications in orthopaedics and traumatology. EFORT Open Rev 2016;1:121-7. [Google Scholar | PubMed]
- 11.Mustahsan VM, Helguero CG, He G, Komatsu DE, Hansen D, Pentyala S, et al. 3D-printed guides in bone tumor resection: Studying their error and determining a safety margin for surgery. Orthopedics 2022;45:169-73. [Google Scholar | PubMed]