Rapid molecular identification of pathogens in prosthetic joint infections enables early targeted therapy and informed surgical decisions, supporting better outcomes and antimicrobial stewardship
Dr. Shreya Chaudhuri, Department of Clinical Microbiology and Infectious Diseases, Yashoda Group of Hospitals, Gaur City 2, Greater Noida - 201301, Uttar Pradesh, India. E-mail: dr.shreyachaudhuri@gmail.com
Introduction: Prosthetic joint infections (PJIs) are a serious complication of arthroplasty, often necessitating prolonged therapy and revision surgeries. Haemophilus influenzae is an exceedingly rare cause of PJI, especially in immunocompromised individuals. Its fastidious nature frequently leads to negative cultures, delaying diagnosis and targeted therapy.
Case Report: We report the case of a 52-year-old male with rheumatoid arthritis on immunosuppressive therapy, who presented with acute right hip pain, swelling, and purulent wound discharge 3 months after revision total hip arthroplasty. Initial workup revealed elevated inflammatory markers and signs of joint infection (JI). Conventional microbiological methods including Gram stain, acid-fast bacilli testing, and enrichment cultures were inconclusive. However, syndromic multiplex polymerase chain reaction (PCR) using the BioFire® JI panel detected H. influenzae within 2 h of intraoperative sample collection. The patient underwent debridement, targeted antibiotic therapy, and implant retention (DAIR). Empirical therapy with meropenem and vancomycin was promptly de-escalated to intravenous ceftriaxone, based on PCR results. Clinical recovery was marked by rapid decline in C-reactive protein, resolution of symptoms, and a successful switch to oral therapy. At 6-week and 16-week follow-up, the patient was asymptomatic, with normal inflammatory markers and radiographs, and a functioning retained implant.
Conclusion: This case underscores the clinical value of rapid syndromic testing in detecting rare, fastidious pathogens like H. influenzae in PJIs. The early identification facilitated timely DAIR, targeted antibiotic therapy, reduced hospital stays, and preserved the implant. The approach highlights the role of molecular diagnostics in enhancing antimicrobial stewardship and improving clinical outcomes in complex orthopedic infections.
Keywords: Prosthetic joint infection, debridement and implant retention, Haemophilus influenzae, syndromic diagnosis, rapid pathogen identification, culture-negative infection.
Prosthetic joint infections (PJIs) are serious complications of joint arthroplasty that can necessitate revision or excision surgeries, prolonged hospitalization, and extended courses of intravenous antibiotics, often resulting in suboptimal functional outcomes. Approximately 1% of hip replacements and 0.7% of knee replacements develop these infections [1]. Although Gram-negative organisms represent a minority of PJI cases, they are notably more challenging to treat and often yield less favorable outcomes [2]. Both the Staphylococcus aureus complex and coagulase-negative Staphylococcus species are recognized as the primary pathogens in PJIs. Notably, Spanish study in 2019 reported a higher prevalence of coagulase-negative staphylococci compared to the S. aureus complex, whereas a contemporaneous French study found the opposite [3,4]. Interestingly, an Indian study conducted at a tertiary care center (Sebastian et al., 2019) revealed a different microbiological landscape. Among patients who underwent hip or knee arthroplasty between 2013 and 2017, the cumulative incidence of PJI was 1.1% – comparable to global estimates. However, Gram-negative aerobes emerged as the most frequently isolated pathogens (61%), a stark contrast to Western data where Gram-positive organisms predominate. Staphylococcus aureus remained the most common individual organism (19.5%), yet the overall pathogen profile highlighted a worrying preponderance of multidrug-resistant (54%) and methicillin-resistant (34%) organisms [5]. Haemophilus influenzae type B (Hib) is a well-known Gram-negative bacterium that can cause osteomyelitis and septic arthritis. Routine childhood immunization against Hib has greatly reduced invasive infections from this bacterium, although the vaccine does not protect against other H. influenzae serotypes or non-typable strains [6]. This case highlights the importance of syndromic testing in prosthetic joint infections to identify the rare pathogens in post total hip replacement infections and initiation of targeted antimicrobial therapy and appropriate surgical decision.
A 52-year-old male presented with severe pain, swelling, and pus discharge from operated wound right hip and fever with chills for 5–6 days. He had undergone a revision total hip replacement on the right side 3 months prior (November 2024), following a primary total hip replacement in 2013. He is a known case of seropositive rheumatoid arthritis and had recently been treated with the immunomodulator injection Golimumab, due to inadequate symptom control with non-steroidal anti-inflammatory drugs and other conventional therapies.
On examination
Patient was febrile. His right hip was swollen, tender with purulent discharge form the post-surgery wound, with a decreased range of active and passive movement secondary to pain in right hip joint. Sensation and pulses were intact in all limbs. Movement of toes of the right limb was present. He was admitted for suspected acute infection of the right hip joint.
Investigations
Hip X-ray shows no fracture, implant properly seated, and no evidence of loosening or osteomyelitis.
Laboratory findings:
- C-reactive protein (CRP) = 320 mg/L (High) (0–5 mg/L)
- Hemoglobin 12.6 g/dL (13–17 g/dL)
- White cell count = 11.34 × 103/mm3 – High (4–10 × 103/mm3)
- Platelet counts – normal
- Random blood sugar = 265 mg/dL.(high)
- The pus sample and tissue collected intraoperatively were sent for syndromic testing with BioFire® joint infection (JI) panel which detected a single pathogen – influenzae within 2 h of sample collection.
- Methodology used for syndromic multiplex polymerase chain reaction (PCR) testing the BioFire® JI panel was utilized. This single-sample, rapid multiplex PCR assay simultaneously detects 39 targets – encompassing Gram-positive and Gram-negative bacteria, yeast, and key antimicrobial resistance (AMR) genes – directly from synovial fluid. Compared to conventional culture, the BioFire® JI Panel is significantly faster and offers improved diagnostic yield, enabling early, and pathogen-guided management that can inform surgical decision-making and shorten time to effective therapy [7,8].
- The Gram stain of the sample shows numerous polymorphs and occasional gram-negative bacteria. However, the cultures of the pus and tissue sample by conventional methods with enrichment show no growth.
- Acid-fast staining and GeneXpert was also done which were negative.
- Histopathology report – Biopsy from the right hip revealed intense-mixed inflammatory cells infiltrate, giving impressions of infective osteoarthritis.
- GeneXpert Mycobacterium tuberculosis with rifampicin.- Negative
Treatment
- Patient was started on empirical antibiotics IV Meropenem and V
- Following this, surgical intervention was done with debridement of wound over the right hip and incision and drainage of abscess over right hip. All dead and necrotic tissue right hip was excised. All dead and necrotic tissue was excised. Vacuum-assisted closure application was done on same day of patient admission. The pus and tissue sample was sent for syndromic testing with BioFire® JI panel, which detected a single pathogen influenzae.
- After this, targeted antibiotic therapy for influenzae was started.
- Initial empirical therapy on admission was I/V Meropenem and Vancomycin; following the detection of influenzae patient was started on targeted therapy with I/V Ceftriaxone 2 g IV 12 hourly by infusion over 3–4 h. Meropenem and Vancomycin were discontinued same day.
- Intravenous ceftriaxone was given to the patient for 2 weeks.
- Patient CRP was monitored regularly which shows gradual decrease. CRP dropped to 11.02 mg/L after 2 weeks of antibiotic therapy and patient was afebrile.
- Targeted IV antibiotic was continued for 2 weeks with monitoring of inflammatory markers which gradually decreased, patient was discharges after 2 weeks, in stable condition on oral ceftriaxone 200 mg twice a day for 4 weeks.
- For this patient, implant was retained.
Patient outcome and follow-up
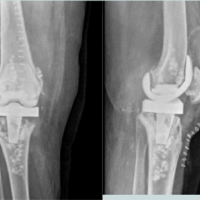
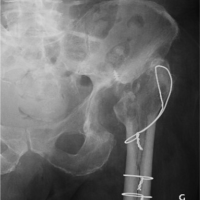
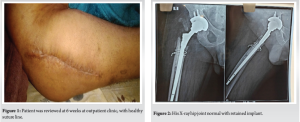
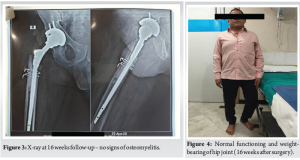
- He completed a total of 6-week course of Ceftriaxone 2 g 12 hourly (2 weeks as an inpatient and 4 weeks as an outpatient). The patient was reviewed at 6 weeks at outpatient clinic, with healthy suture line (Fig. 1) and all inflammatory markers were normal. His X-ray hip joint (Fig. 2) normal with retained implant. The clinical examination of hip was normal. There was no evidence of clinical, radiological, or microbiological treatment failure at follow-up. He came for follow-up at orthopedic outpatient department and the X-ray (Fig. 3) shows the X-ray hip joint with no evidence of bony involvement, no lysis or periosteal reaction suggestive of osteomyelitis and hip joint can bear weight normally (Fig. 4). He will be followed up at 8 months and 12 months to ensure no relapse.
According to the 2024 Annual Report, there has been a steady increase in the number of primary joint replacement procedures over the past decade. In 2024 alone, more than 1.5 million primary hip replacements and approximately 116,845 primary knee replacements were performed in England [9]. India is projected to experience the highest growth rate in joint replacement procedures globally between 2020 and 2026. This trend is evident from the increase in the number of knee replacement procedures reported to the national registry, which rose from 1019 cases in 2006 to approximately 27,000 cases in 2019 [10]. Peri-PJI is a unique clinical entity, markedly different from infections involving native bones or joints. PJI arises from a complex host-microbe interaction, where even a minimal bacterial or occasionally fungal, this load can adhere to prosthetic surfaces and form biofilms, triggering infection [11,12]. Study by Rakow et al. indicates that revision arthroplasties are associated with higher risk of hematogenous infection than primary implants [13]. Study by Iqbal et al., shows management of periprosthetic joint infection which is 4.5 times more expensive than uneventful primary total knee arthroplasty (TKA) [14]. When identified early, acute hematogenous prosthetic joint infections can often be effectively managed with debridement and implant retention (DAIR). Haemophilus species are uncommon but well-documented causes of PJIs, particularly in patients with underlying immunosuppression or comorbidities. Early literature includes Limbird’s 1985 report of H. influenzae PJI in a 63-year-old woman, successfully managed with debridement and antibiotics while retaining the prosthesis [15]. More recent case series underscore the diversity of Haemophilus pathogens and clinical contexts:
H. influenzae in chronic lymphocytic leukemia
A 71-year-old male with chronic lymphocytic leukemia presented with a hot, swollen knee; cultures grew non-encapsulated H. influenzae. After two surgical washouts and a switch from piperacillin–tazobactam to amoxicillin, followed by 6 weeks of IV ceftriaxone, he achieved full recovery [6].
Haemophilus parainfluenzae in a diabetic TKA patient
A 67-year-old male with diabetes and hypertension developed knee PJI 1-year post-TKA. Maldi-time-of-flight mass spectrometry identified H. parainfluenzae, which was resistant to ampicillin/clarithromycin but sensitive to ceftriaxone and ciprofloxacin. A one-stage revision with targeted IV then oral therapy led to full recovery at 3 years [16].
Non-typeable H. influenzae in rheumatoid arthritis
A 60-year-old woman on immunosuppressants developed PJI after a fall and pneumonia. Despite negative cultures, joint fluid PCR confirmed H. influenzae, guiding explanation, debridement, and prolonged IV antibiotics [17]. Early identification of hematogenous PJI within the first 3 weeks of symptom onset enables successful debridement with prosthesis retention, regardless of implant age. Preserving fixed components offers key patient benefits, including single-stage surgery, reduced blood and bone loss, minimal soft-tissue disruption, and lower perioperative risk [13]. In the present study, alongside prompt clinical evaluation, rapid microbiological identification was achieved within hours of admission using a syndromic approach with the BioFire® multiplex PCR panel. This enabled confirmation of H. influenzae-associated PJI of the right hip as early as 2–3 h, facilitating timely surgical intervention and initiation of targeted, streamlined antimicrobial therapy. Due to such early and precise intervention, the hip implant was successfully retained, contributing to a favorable clinical outcome. PJIs due to unsuspected and rare pathogens like Haemophilus species present unique diagnostic and therapeutic challenges. Their fastidious growth characteristics often render conventional cultures insensitive, resulting in delayed diagnosis and prolonged empiric broad-spectrum therapy. Syndromic multiplex PCR platforms – exemplified by the BioFire® JI panel – overcome this limitation by delivering pathogen identification within hours, thereby enabling precision antimicrobial stewardship. In our 52-year-old patient on Golimumab, routine cultures (including enrichment), acid-fast bacilli staining, and GeneXpert were uniformly negative. In contrast, the BioFire® panel detected H. influenzae in intraoperative synovial fluid in under 2 h. Armed with this definitive result, the team de-escalated immediately from meropenem plus vancomycin to ceftriaxone monotherapy. The benefits were multifold:
- Implant retention and cost-effectiveness
- Factors that are known to influence the DAIR outcome in the management of PJIs include the timing and numbers of debridement, the components’ exchange, the causative microorganism/s, and the duration of antibiotic treatment [18]. Early, pathogen-directed therapy permitted a DAIR approach rather than full prosthesis revision. DAIR not only preserves native implants – avoiding the substantial costs and morbidity of revision arthroplasty – but also minimizes operating room time and the need for additional ancillary diagnostics that often accompany culture-negative PJIs.
- Reduced adverse events and ancillary costs
- By discontinuing Vancomycin and Meropenem, we averted risks of acute kidney injury, “Red Man” infusion reactions, and meropenem-associated clostridium difficile infection. Avoidance of these treatment-emergent adverse events (TEAEs) further reduces hospitalization costs and resource utilization.
- Shortened length of stay
- Ceftriaxone’s once-daily dosing and elimination of therapeutic drug monitoring facilitated earlier discharge planning. In this case, the patient achieved clinical and biomarker (CRP) resolution within 2 weeks of IV therapy, followed by a 4-week oral course, without complications.
- Optimized antimicrobial stewardship and AMR mitigation
- Narrowing therapy to ceftriaxone reduced broad-spectrum antibiotic exposure, thereby decreasing selective pressure for resistant organisms. Rapid molecular diagnostics thus function as an antimicrobial stewardship tool, ensuring appropriate, targeted regimens, and preserving the efficacy of critical agents.
- Improved patient outcomes
- Early identification and tailored therapy not only controlled infection effectively but also preserved prosthesis function, minimized perioperative morbidity, and improved recovery trajectories compared to the morbidity and prolonged rehabilitation associated with two-stage revision surgeries.
By integrating rapid syndromic testing with a proactive DAIR protocol, this case illustrates how advanced diagnostics can transform PJI management. The BioFire® JI panel not only unmasked an unexpected, hard-to-culture pathogen but also empowered a cost-effective, patient-centered strategy that reduced TEAEs, shortened hospitalization, curtailed unnecessary antimicrobial use, and ultimately enhanced clinical outcomes while addressing the global imperative of AMR.
Rapid pathogen identification in prosthetic joint infection achieved within hours compared to the 2–3 day delay with conventional cultures facilitates timely surgical decision-making and initiation of targeted antimicrobial therapy. In this case, early diagnosis enabled implant retention, and at 8-week follow-up, the patient showed no clinical signs of infection with normalization of inflammatory markers, underscoring the clinical impact of this approach and its contribution to antimicrobial stewardship.
This case highlights the critical role of rapid molecular diagnostics in prosthetic joint infections, particularly when conventional cultures are inconclusive. The use of syndromic PCR enabled early identification of H. influenzae – a rare, fastidious pathogen – within hours, guiding timely surgical debridement and initiation of targeted antimicrobial therapy. In time-sensitive scenarios such as acute PJIs, early pathogen detection is essential for optimizing outcomes, enabling implant retention, and strengthening antimicrobial stewardship efforts.
References
- 1.Moran E, Byren I, Atkins BL. The diagnosis and management of prosthetic joint infections. J Antimicrob Chemother 2010;65:ii45-54. [Google Scholar | PubMed]
- 2.Moran E, Masters S, Berendt AR, McLardy-Smith P, Byren I, Atkins BL. Guiding empirical antibiotic therapy in orthopaedics: The microbiology of prosthetic joint infection managed by debridement, irrigation and prosthesis retention. J Infect 2007;55:1-7. [Google Scholar | PubMed]
- 3.Benito N, Mur I, Ribera A, Soriano A, Rodríguez-Pardo D, Sorlí L, et al. The different microbial etiology of prosthetic joint infections according to route of acquisition and time after prosthesis implantation, including the role of multidrug-resistant organisms. J Clin Med 2019;8:673. [Google Scholar | PubMed]
- 4.Triffault-Fillit C, Ferry T, Laurent F, Pradat P, Dupieux C, Conrad A, et al. Microbiologic epidemiology depending on time to occurrence of prosthetic joint infection: A prospective cohort study. Clin Microbiol Infect 2019;25:353-8. [Google Scholar | PubMed]
- 5.Sebastian S, Malhotra R, Sreenivas V, Kapil A, Chaudhry R, Dhawan B. A clinico-microbiological study of prosthetic joint infections in an Indian tertiary care hospital: Role of universal 16S rRNA gene polymerase chain reaction and sequencing in diagnosis. Indian J Orthop 2019;53:646-54. [Google Scholar | PubMed]
- 6.Khan S, Reddy S. Haemophilus influenzae infection of a prosthetic knee joint in a patient with CLL: A vaccine preventable disease. BMJ Case Rep 2013;2013:bcr2013010307. [Google Scholar | PubMed]
- 7.Hoffman T, Kriger O, Cohen S, Gefen-Halevi S, Yahav D, Amit S. Real-life experience and diagnostic utility of the biofire joint infection PCR panel in bone and joint infections: Analysis of a prospective validation study. Infect Dis Ther 2023;12:1437-43. [Google Scholar | PubMed]
- 8.Berinson B, Spenke L, Krivec L, Tanida K, Both A, Keller J, et al. Performance and hypothetical impact on joint infection management of the biofire joint infection panel: A retrospective analysis. J Clin Microbiol 2023;61:e0059223. [Google Scholar | PubMed]
- 9.National Joint Registry. 21st NJR Annual Report; 2024. [Google Scholar | PubMed]
- 10.Vaidya SV, Jogani AD, Pachore JA, Armstrong R, Vaidya CS. India joining the world of hip and knee registries: Present status-a leap forward. Indian J Orthop 2020;55 Suppl 1:46-55. [Google Scholar | PubMed]
- 11.Salar O, Phillips J, Porter R. Diagnosis of knee prosthetic joint infection; aspiration and biopsy. Knee 2021;30:249-53. [Google Scholar | PubMed]
- 12.Wagenaar FB, Löwik CA, Zahar A, Jutte PC, Gehrke T, Parvizi J. Persistent wound drainage after total joint arthroplasty: A narrative review. J Arthroplasty 2019;34:175-82. [Google Scholar | PubMed]
- 13.Rakow A, Perka C, Trampuz A, Renz N. Origin and characteristics of haematogenous periprosthetic joint infection. Clin Microbiol Infect 2019;25:845-50. [Google Scholar | PubMed]
- 14.Iqbal F, Shafiq B, Noor SS, Ali Z, Memon N, Memon N. Economic burden of periprosthetic joint infection following primary total knee replacement in a developing country. Clin Orthop Surg 2020;12:470-6. [Google Scholar | PubMed]
- 15.Limbird TJ. Hemophilus influenzae infection of a total hip arthroplasty. Clin Orthop Relat Res 1985;199:182-4. [Google Scholar | PubMed]
- 16.Medel-Plaza M, Auñón A, Blanco A, García-Cañete J, Salar-Vidal L, Esteban J. Periprosthetic joint infection caused by Haemophilus parainfluenzae. Case report and literature review. Rev Esp Quimioter 2023;36:325-8. [Google Scholar | PubMed]
- 17.Weenders S, Heller KD, Krueger DR. Haemophilus influenzae infection of a prosthetic knee joint in a patient with rheumatoid arthritis: A case report. Orthopadie (Heidelb) 2023;52:843-7. [Google Scholar | PubMed]
- 18.Schiavi P, Pogliacomi F, Calderazzi F, Domenichini M, Ceccarelli F, Vaienti E. Dair approach in 7 infected total hip arthroplasties: Our experience and current concepts of the literature. Acta Biomed 2022;92:e2021572. [Google Scholar | PubMed]