This article highlights the importance of careful diagnostic workup for neuropathic pain, including the use of therapeutic and diagnostic ultrasound-guided hydrodissection.
Dr. Keith T. Aziz, Department of Orthopedic Surgery, Mayo Clinic Florida, Jacksonville, Florida. E-mail: aziz.keith@mayo.edu
Introduction: Neuropathic pain following trauma is a relatively common occurrence; however, diagnostic workup and treatment can be difficult. We present a case of chronic neuropathic pain in which surgical exploration and neurolysis yielded complete remission of pain. Histopathology revealed an array of findings, including evidence of fat necrosis with calcification, granuloma formation, and a foreign body, representing a particularly complex etiology for the patient’s symptoms that has not, to our knowledge, been previously reported in the literature.
Case Report: A 31-year-old female presented to our clinic with chronic neuropathic pain after sustaining a blunt injury to the medial aspect of the right leg 15 years previously while playing softball. Her pain was localized to the anteromedial lower leg and was not relieved by medical management and therapy. She temporarily had complete relief of her symptoms with ultrasound-guided hydrodissection. She was treated with surgical exploration and neurolysis, and postoperatively, the patient had no complications and complete relief of her pain.
Conclusion: We present a case of a 31-year-old patient who had perineural scarring and chronic neuropathic pain necessitating surgical exploration and neurolysis. Our case highlights the importance of investigating the etiology of neuropathic pain with several modalities and demonstrates the utility of ultrasound-guided peripheral nerve hydrodissection for both therapeutic and diagnostic purposes.
Keywords: Neurolysis, ultrasound, hydrodissection, neuropathic pain, fat necrosis, foreign body, granuloma.
Chronic pain is a relatively common sequela following trauma or surgical intervention to peripheral nerves [1,2]. This pain may be persistent and lead to a decrease in the quality of life for the patient. The pathophysiology of this pain can be related to traumatic neuroma formation, in which damaged perineurium leads to dysregulated regeneration with myofibroblasts and Schwann cells often found within scar tissue [3]. In other cases, symptoms may manifest primarily due to nerve compression from adjacent structures, such as hematoma, bony abnormalities, and ganglion cysts [4,5]. Chronic compression leads to a complex array of reactions that may contribute to symptoms, including demyelination and remyelination of Schwann cells and fibrosis of the surrounding perineurium [6,7]. Rare reported compressive causes include fat necrosis and foreign body reaction. Fat necrosis, in which lysed adipose cells release triglycerides that saponify with nearby calcium, has been reported in cases of ulnar and radial neuropathies [8,9]. Foreign body reaction, a chronic inflammatory process involving macrophage recruitment, has been implicated in case reports demonstrating neuropathies from a spinal cord stimulator and an annular closure device [10,11]. Here, we report a case of chronic and recurrent neuropathic pain following trauma in a patient with a constellation of histopathologic findings, including fat necrosis with calcification, local scar tissue formation, and presence of foreign body material. The patient was informed that data concerning the case would be submitted for publication, and she provided consent.
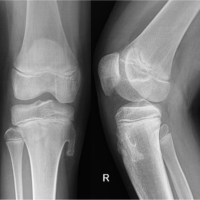
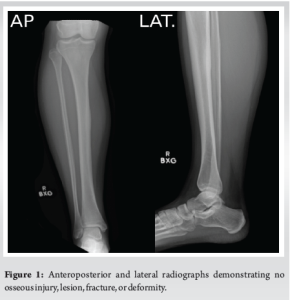
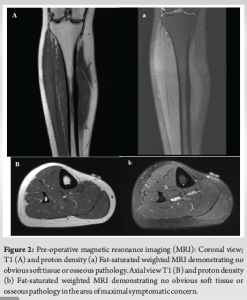
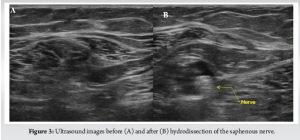
A 31-year-old female presented with chronic right lower leg pain that began after a blunt injury 15 years ago to the anteromedial aspect of her lower leg. She was struck in the anteromedial aspect of her lower leg by a softball and had pain, but did not immediately seek evaluation. Because of continued pain for the next 2 months, she underwent evaluation by an outside orthopedic surgeon and was found to have a stress reaction but no fracture. This was treated conservatively with non-steroidal anti-inflammatories, but she had continued pain localized to the anteromedial lower limb. The pain was described as a constant burning discomfort with extreme sensitivity to light touch and water. There was no color change, swelling, or proximal spread. She had undergone multiple sessions of topical and systemic neuropathic pain treatment with minimal relief. She was referred to pain management, where radiographic evaluation and advanced imaging demonstrated no fracture, osseous irregularity, periosteal reaction, or osseous lesions were present (Fig. 1). Magnetic resonance imaging (MRI) demonstrated no fracture, osseous irregularity, or apparent soft-tissue injury (Fig. 2). She ultimately underwent diagnostic ultrasound, which demonstrated atypical signal around the saphenous nerve (Fig. 3). She subsequently underwent hydrodissection of the saphenous nerve for therapeutic and diagnostic purposes, and had complete relief of her pain (Fig. 3). She also underwent serial ultrasound guided hydrodissection of the saphenous nerve, with the aim of isolating it from surrounding adhesions and scar tissue. Unfortunately, the duration of symptomatic relief shortened over time and did not lead to sustained improvement in her pain. Because she had refractory symptoms, she was referred to our peripheral nerve clinic.
On examination, there was a well-healed scar over the anteromedial aspect of the middle third of the tibia. She had significant hyperesthesia and allodynia about the scar, with a positive Tinel’s sign reproducing the burning pain radiating distally. No signs of proximal nerve compression were present.
After a discussion about different options, and because she failed non-surgical measures, the patient engaged in shared decision-making and elected to proceed with surgical exploration with saphenous nerve neurolysis. On the day of surgery, our regional anesthesiology team performed an ultrasound and identified the saphenous nerve and atypical perineural tissue. The area was marked with an indelible marker to localize the nerve and site of increased perineural signal. A 4 cm longitudinal incision was made over the anteromedial mid-tibia in line with the markings made with ultrasound assistance. A small, firm, pale nodular mass was found adherent to the deep fascia and saphenous nerve.
Using microsurgical techniques, the nerve was carefully dissected free from the scar tissue, which was tightly adherent to the nerve and fascia. The nerve appeared thinned at the compression site but remained intact, without obvious neuroma. After neurolysis, the nerve was free of compression and could be tracked proximally and distally (Fig. 4). The scar mass and a small margin of fascia were excised and sent for pathology and culture. At her 2-week follow-up, her burning pain had significantly diminished, and Tinel’s sign was absent in follow-up evaluations. She ultimately had complete relief of her pain and resumed normal activities without post-operative complications.
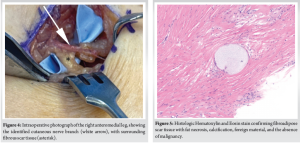
Pathology confirmed a chronic post-traumatic scar. Grossly, the specimen was fibrofatty tissue with calcifications. Microscopically, it showed fibroadipose tissue with fat necrosis, granulomas, foreign material, calcification, and fibrosis, negative for malignancy. No neuroma was identified. Findings suggested the presence of foreign material residual or bone fragments from the original injury as a possible etiology of the excessive fibroglandular tissue formation (Fig. 5).
This case report illustrates a rare cause of chronic leg pain secondary to nerve entrapment that manifested years following the initial trauma. Traumatic neuropathic pain can persist even after the initial trauma has recovered. In our patient, although the tibial stress reaction had resolved, the saphenous nerve caused persistent neuropathic symptoms. The diagnosis was challenging because initial MRI results were inconclusive, showing neither neuroma nor clear nerve compression. Clinical suspicion was therefore largely based on the neuropathic nature of the pain. The initial injury was significant enough to cause a tibial stress reaction, though the patient could not definitively recall if the skin barrier was disrupted at the inciting event. The final pathology results revealed the presence of foreign bodies, which suggests that some debris was embedded and may have contributed to the excessive formation of perineural fibroglandular tissue. Our case has similarities with a case of saphenous nerve entrapment reported by Haraguchi et al. [12]. Similar to our case, Haraguchi et al. described non-specific imaging characteristics and emphasized that correct diagnosis relies on history and clinical suspicion. Haraguchi et al. explicitly recommended the use of diagnostic local anesthetic blocks as both a diagnostic and a therapeutic intervention. Similarly, diagnosis and temporary relief of symptoms in our patient were made through ultrasound-guided injections of anesthetic, and the utility of this minimally invasive diagnostic procedure is illustrated. Haraguchi et al. also found that surgical neurolysis could produce substantial and sustained relief, consistent with the favorable outcomes following microsurgical neurolysis in our case. Our case is distinct in that the patient described by Haraguchi et al. had undergone prior surgical intervention with intramedullary nailing, which places patients at increased risk for post-operative nerve injury and perineural scarring. Yao et al. reviewed a series of patients with traumatic peripheral nerve injuries and highlighted the variability of presentation and outcomes in this patient population [13]. Their review illustrates how conservative management typically only provides transient relief, as in our case, and highlights the benefit of tailored surgical techniques based on the specific needs of individual patients and intraoperative findings. By employing a variety of imaging modalities and procedural interventions, we were able to ensure symptomatic relief in our case.
This case demonstrates the diagnostic difficulty and treatment challenge of chronic neuropathic pain due to peripheral nerve entrapment following blunt trauma. This case highlights the importance of having a high index of clinical suspicion and the employment of diagnostic modalities such as ultrasound-guided injections when advanced imaging is inconclusive. Individualized surgical management, guided by intraoperative findings, eventually led to prolonged symptomatic relief in our patient, highlighting the importance of utilizing these individualized treatments in the management of traumatic peripheral nerve injury.
This case highlights the importance of careful diagnosis and demonstrates the utility of ultrasound and ultrasound-guided hydrodissection in the diagnostic algorithm of peripheral nerve pain. It also demonstrates the importance of adherence to fundamental soft-tissue principles with meticulous dissection for surgical management to decrease the likelihood of recurrent neuropathic pain.
References
- 1. Keene DJ, Knight R, Bruce J, Dutton SJ, Tutton E, Achten J, et al. Chronic pain with neuropathic characteristics after surgery for major trauma to the lower limb: Prevalence, predictors, and association with pain severity, disability, and quality of life in the UK WHiST trial. Bone Joint J 2021;103-B:1047-54. [Google Scholar] [PubMed]
- 2. Ciaramitaro P, Mondelli M, Logullo F, Grimaldi S, Battiston B, Sard A, et al. Traumatic peripheral nerve injuries: Epidemiological findings, neuropathic pain and quality of life in 158 patients. J Peripher Nerv Syst 2010;15:120-7. [Google Scholar] [PubMed]
- 3. Zabaglo M, Dreyer MA. Neuroma. In: StatPearls. Treasure Island, FL: StatPearls Publishing, LLC; 2025. [Google Scholar] [PubMed]
- 4. Steinfeldt T, Wiesmann T, Nimphius W, Cornelius V, Eismann D, Kratz T, et al. Perineural hematoma may result in nerve inflammation and myelin damage. Reg Anesth Pain Med 2014;39:513-9. [Google Scholar] [PubMed]
- 5. Martinoli C, Bianchi S, Gandolfo N, Valle M, Simonetti S, Derchi LE. US of nerve entrapments in osteofibrous tunnels of the upper and lower limbs. Radiographics 2000;S199-213; discussion S213-7. [Google Scholar] [PubMed]
- 6. Gupta R, Rummler L, Steward O. Understanding the biology of compressive neuropathies. Clin Orthop Relat Res 2005;436:251-60. [Google Scholar] [PubMed]
- 7. Prinz RA, Nakamura-Pereira M, De-Ary-Pires B, Fernandes D, Fabião-Gomes BD, Martinez AM, et al. Axonal and extracellular matrix responses to experimental chronic nerve entrapment. Brain Res 2005;1044:164-75. [Google Scholar] [PubMed]
- 8. Hwang HJ, Lee GS, Park HK, Chang JC. Radial nerve compression caused by traumatic fat necrosis. Nerve 2017;3:67-70. [Google Scholar] [PubMed]
- 9. Segal R, Saito R, Sen C. Ulnar neuropathy caused by fat necrosis with cystic myxoid degeneration at the upper forearm: Case report. Neurosurgery 1988;23:669-71. [Google Scholar] [PubMed]
- 10. Lennarson PJ, Guillen FT. Spinal cord compression from a foreign body reaction to spinal cord stimulation: A previously unreported complication. Spine (Phila, Pa 1976) 2010;35:E1516-9. [Google Scholar] [PubMed]
- 11. McClure JJ, Jentoft ME, Sandhu SS, Chen SG, Abode-Iyamah KO. Bone-anchored annular closure device leading to histiocytic-inflammation-induced neuropathy with resolution after removal: A case report. Eur Spine J 2024;33:2840-5. [Google Scholar] [PubMed]
- 12. Haraguchi T, Kume S, Jimbo K, Gotoh M, Shiba N, Okawa T. Saphenous nerve entrapment neuropathy after closed tibial fracture: A case report. JBJS Case Connect 2021 Apr 9;11(2). doi: 10.2106/JBJS.CC.20.00670. [Google Scholar] [PubMed] [CrossRef]
- 13. Yao C, Zhou X, Zhao B, Sun C, Poonit K, Yan H. Treatments of traumatic neuropathic pain: A systematic review. Oncotarget 2017;8:57670-9. [Google Scholar] [PubMed]