Our case demonstrates a novel approach to manage the uncommon combination of GCTB recurrence and infection through a single intervention, employing antibiotic-loaded bone cement for infection control and extended curettage for comprehensive neoplastic cell removal from surrounding bone tissue.
Dr. Harsha Vardhan Reddy, Department of Orthopedics, All India Institute of Medical Sciences, Nagpur, Maharashtra, India. E-mail: m.harshareddy1998@gmail.com
Introduction: Giant cell tumor of bone (GCTB) ranks among the most prevalent locally aggressive tumor lesions, displaying a diverse range of biological behaviors. Recurrence of GCTB is well-documented, often attributed to microscopic tumour remnants remaining after intralesional curettage, with increased concern when infection occurs postoperatively. Studies suggest the limited effectiveness of adjuvants in preventing giant cell tumour recurrence, emphasizing the necessity of complete removal of malignant cells. We describe our experience with a rare presentation of recurrence together with infection in an operated case of GCTB right proximal tibia with curettage with cementing and plate stabilization.
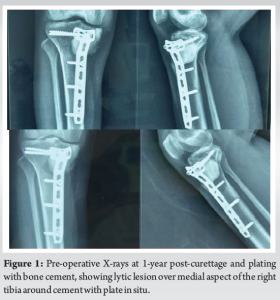
Case Report: A 28 year-old female who was operated case of GCTB right proximal tibia with curettage with cementing and plate stabilization in 2020 presented to our outpatient department with complaints pain in right leg while walking for 3 months in October 2021. Plain radiography of the right knee suggestive of lytic lesion over medial aspect of the right tibia around cement with plate in situ and magnetic resonance imaging right knee suggestive of recurrence of the tumor with no breach in joint line and no involvement of neurovascular structures. The patient was planned for implant removal with extended curettage and plate stabilization and cementing with antibiotics (vancomycin and gentamycin) after sending tissues for culture sensitivity and histopathology. Intraoperative cultures showed growth of methicillin-sensitive staphylococcus aureus which was sensitive to cefoxitin, gentamicin, cotrimoxazole, and doxycycline following which pt received 2 weeks of intravenous antibiotics and 4 weeks of oral antibiotics as per culture sensitivity with no post-operative wound complications and had satisfactory functional outcome. The patient was followed at regular intervals till 2-year follow-up did not show any signs of recurrence and infection.
Conclusion: The manifestation of GCTB recurrence alongside infection is exceedingly rare with limited literature evidence. Our case illustrates a method to address both issues concurrently during a single procedure, utilizing antibiotic bone cement to tackle infection and extended curettage for thorough removal of neoplastic cells from the surrounding bone.
Keywords: Giant cell tumor, recurrence, infection.
Giant cell tumor of bone (GCTB) is a prevalent benign bone tumor characterized by multinuclear giant cells and a notable tendency for local recurrence. It constitutes 20% of benign skeletal tumors and 5% of primary bone tumors [1] typically affecting individuals aged between the third and fifth decades, with a slight female predominance. While it can develop in any bone, it predominantly arises in the metaphysis extending into the epiphysis of long bones, particularly in the distal femur, proximal tibia, and distal radius. In the axial spine, the sacrum is the most common site [2]. Patients with GCTB typically present with pain, swelling, joint effusion, and limitations in weight-bearing and joint range of motion, often leading to pathological fractures as cortical bone thinning occurs [2].
GCTB lesions are classified using the Campanacci grading method, where Grade I lesions are confined to the bone, Grade II lesions show cortical enlargement without perforation, and Grade III lesions exhibit soft tissue extension and cortical perforation [3]. Despite its benign nature, giant cell tumor (GCT) can display local aggressiveness, particularly in Grade II and III tumors, leading to a high recurrence rate. In addition, about 1% of benign GCTs can metastasize hematogenous, with the lungs being the most common site [4].
In this report, we discuss the management of a unique presentation of GCTB recurrence along with infection which was finally managed with implant removal with extended curettage and plate stabilization and polymethylmethacrylate (PMMA) cementing with antibiotics (vancomycin and gentamycin) addressing both infection and neoplastic recurrence in a single stage.
A 28-year-old female, previously treated for GCTB of the right proximal tibia with curettage, cementing, and plate stabilization in 2020, presented to our outpatient department with complaints of pain in right leg while walking since October 2021. There was no history of trauma and no associated fever, weight loss, or other constitutional symptoms. Local examination revealed tenderness over the anteromedial aspect of the proximal tibia with a local rise in temperature and a single healed previous surgical scar with no discharge, accompanied by painful terminal restriction of knee range of motion. Plain radiography of right knee was suggestive of lytic lesion over medial aspect of the right tibia around cement with plate in situ and magnetic resonance imaging (MRI) right knee was suggestive of recurrence of the tumor with no breach in joint line and no involvement of neurovascular structures (Fig. 1).
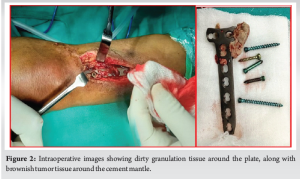
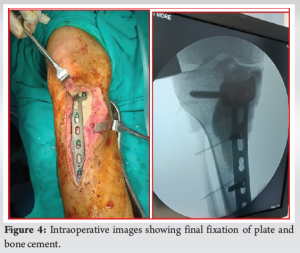
The patient underwent implant removal with debridement, extended curettage, cementing, and plate stabilization. Intraoperatively, dirty granulation tissue was observed around the plate, along with brownish tumor tissue around the cement mantle extending laterally, proximally, and distally (Fig. 2). Tissue samples were collected for bacterial culture sensitivity and histopathological analysis (Fig. 3). Debridement and removal of tumor tissue were performed using bone curettes, followed by chemical curettage with 70% phenol. The remnant cavity was then filled with gel foam, bone cement mixed with 4 g of vancomycin and 160 mg of gentamycin and later stabilized with a 4.5 locking T-buttress plate on the anteromedial aspect of the proximal tibia (Fig. 4).
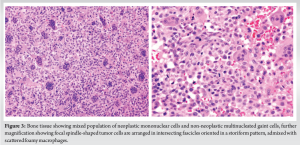
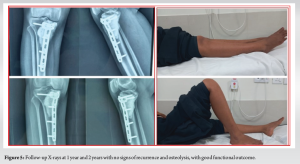
Intraoperative cultures revealed the growth of methicillin-sensitive staphylococcus aureus, sensitive to cefoxitin, gentamicin, cotrimoxazole, and doxycycline. The patient received 2 weeks of intravenous antibiotics followed by 4 weeks of oral antibiotics as per culture sensitivity, experiencing no postoperative wound complications and achieving a satisfactory functional outcome. Histopathology confirmed recurrence of GCTB, exhibiting characteristic multinucleated giant cells and neoplastic mononuclear cells surrounded by bone matrix. The patient was followed at regular intervals for 2 years with no signs of recurrence or infection (Fig. 5).
Recurrence of GCT is a common complication, often emerging within 2 years of the primary treatment. The recurrence rate is notably higher with curettage followed by cementation or bone grafting compared to en bloc resection, as reported by Campanacci et al. [3], who documented a 27% recurrence rate with intralesional procedures. Li et al. proposed that being female, younger in age, having an extraosseous tumor mass, using denosumab, and undergoing curettage are factors that could potentially increase the risk of local recurrence [5]. Diagnosis of GCTB necessitates radiography, computed tomography (CT), and MRI is employed to evaluate the primary tumor. While plain radiography and CT are valuable for assessing the bony component of the tumor, MRI is preferable for evaluating intramedullary and soft tissue extension. Detecting pulmonary metastases, which may occur in a small percentage of patients, necessitates a chest radiograph. Bone scans are useful for identifying additional skeletal disease areas [6]. Histopathological diagnosis was gold standard.
The primary approach to treating GCTB has predominantly relied on surgical methods, sometimes supplemented with additional therapies. The specific surgical procedure chosen typically depends on the Campanacci grade of the tumor and its location within the bone. Curettage and adjuvant therapy are recommended for Campanacci grade 1 or 2 tumors, while tumors with soft tissue extension (Campanacci grade 3) warrant extensive local excision to mitigate their elevated recurrence risk [3]. Intralesional curettage is a standard treatment for GCT, using bone grafting/PMMA cement [7]. Large juxta-articular lesions pose challenges for bone grafting due to limited graft availability, insufficient immediate subchondral strength, and inherent risks of local infection [8]. PMMA cement, in addition to its role in defect reconstruction, is thought to function as an adjuvant through its exothermic reaction and toxic effects on tumor cells, potentially reducing the rate of recurrence according to certain studies [9] with a disadvantage of articular cartilage damage and early onset of joint degeneration [10].
Mahdal et al. in their retrospective study observed that GCTB at proximal tibia location itself was risk factor for recurrence [11]. According to a Scandinavian Sarcoma Group study, 14% of GCT patients experienced at least one local recurrence, with around two-thirds of those cases effectively managed through curettage and cementing [12]. Our case was having the first recurrence of GCTB proximal tibia so we chose extended curettage and cementing with plate stabilization. In our case, the incorporation of antibiotics (vancomycin and gentamycin) with PMMA cement offered an added advantage by delivering a high concentration of local antibiotics to combat infection. Postoperatively, the patient has had a pain-free and acceptable functional range of motion of the knee without any recurrence till 2-year follow-up. Our case was exceedingly rare as no such cases have been reported in available literature as per our knowledge. In medical research concerning infections of tumor prostheses implanted after bone tumor removal, the highest risk of infection leading to limb amputation occurs after resection of bone tumors in the proximal tibia, attributed to the poor condition of soft tissue surrounding the area. Regardless of where the tumor prosthesis is implanted, the approach to managing infections remains largely consistent. Typically, it involves several attempts at debridement (removing infected tissue) along with antibiotic therapy. However, if these measures are unsuccessful, the standard procedure involves removing the infected implant and replacing it with a new one. This replacement can be done in either a one- or two-stage procedure, with the latter showing a decreased risk of failure [13]. Some of the issues that surgeons need to address include multiple surgeries over an extended period, and the patients’ immune system that are weakened due to treatments. Moreover, the extensive exposure of tissues during these surgeries, along with dissection across vascular distributions, adds to the heightened risk of infection (Table 1).
The simultaneous occurrence of GCTB recurrence and infection is a seldom-seen phenomenon with limited literature backing. Our case offers insight into a unified approach to tackle both issues in a single procedure, employing antibiotic mixed PMMA bone cement to combat infection and extended curettage to thoroughly eradicate neoplastic cells from the surrounding bone tissue.
The occurrence of GCTB recurrence together with infection is highly uncommon, with scarce literature supporting such cases. Our report highlights a novel approach to manage both challenges simultaneously in a single intervention, employing antibiotic-infused bone cement to combat infection and extended curettage to ensure comprehensive removal of neoplastic cells from the adjacent bone tissue.
References
- 1.Merchán N, Yeung CM, Garcia J, Schwab JH, Raskin KA, Newman ET, et al. Primary and metastatic bone tumors of the patella: Literature review and institutional experience. Arch Bone Jt Surg 2022;10:190-203. [Google Scholar | PubMed]
- 2.Basu Mallick A, Chawla SP. Giant cell tumor of bone: An update. Curr Oncol Rep 2021;23:51. [Google Scholar | PubMed]
- 3.Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am 1987;69:106-14. [Google Scholar | PubMed]
- 4.Agrawal AC, Choudhary R, Verma S. The successful management of a repetitively infected recurrent proximal humerus giant cell tumour of 20 years’ duration with two-staged surgery: A rare case report. Cureus 2021;13:e14492. [Google Scholar | PubMed]
- 5.Li Z, Müller R, Ruffoni D. Bone remodeling and mechanobiology around implants: Insights from small animal imaging. J Orthop Res 2018;36:584-93. [Google Scholar | PubMed]
- 6.Mendenhall WM, Zlotecki RA, Scarborough MT, Gibbs CP, Mendenhall NP. Giant cell tumor of bone. Am J Clin Oncol 2006;29:96-9. [Google Scholar | PubMed]
- 7.Montgomery C, Couch C, Emory CL, Nicholas R. Giant cell tumor of bone: Review of current literature, evaluation, and treatment options. J Knee Surg 2019;32:331-6. [Google Scholar | PubMed]
- 8.Frassica FJ, Sim FH, Pritchard DJ, Chao EY. Subchondral replacement: A comparative analysis of reconstruction with methyl methacrylate or autogenous bone graft. Chir Organi Mov 1990;75:189-90. [Google Scholar | PubMed]
- 9.Prosser GH, Baloch KG, Tillman RM, Carter SR, Grimer RJ. Does curettage without adjuvant therapy provide low recurrence rates in giant-cell tumors of bone? Clin Orthop Relat Res 2005;435:211-8. [Google Scholar | PubMed]
- 10.Bini SA, Gill K, Johnston JO. Giant cell tumor of bone. Curettage and cement reconstruction. Clin Orthop Relat Res 1995;321:245-50. [Google Scholar | PubMed]
- 11.Mahdal M, Tomáš T, Apostolopoulos V, Adámková D, Múdry P, Staniczková Zambo I, et al. Proximal tibia tumour location and curettage are major risk factors of local recurrence in giant cell tumour of bone. Cancers (Basel) 2023;15:4664. [Google Scholar | PubMed]
- 12.Vult von Steyern F, Bauer HC, Trovik C, Kivioja A, Bergh P, Holmberg Jörgensen P, et al. Treatment of local recurrences of giant cell tumour in long bones after curettage and cementing. A Scandinavian Sarcoma Group study. J Bone Joint Surg Br 2006;88:531-5. [Google Scholar | PubMed]
- 13.Graci C, Maccauro G, Muratori F, Spinelli MS, Rosa MA, Fabbriciani C. Infection following bone tumor resection and reconstruction with tumoral prostheses: A literature review. Int J Immunopathol Pharmacol 2010;23:1005-13. [Google Scholar | PubMed]