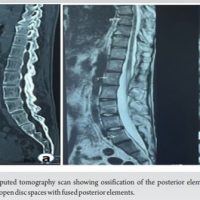
In patients with Ankylosing Spondylitis, Andersson Lesion is an uncommon aseptic disco vertebral lesion that develops as a late consequence. It is crucial to distinguish this condition from infectious spondylodiscitis and metastatic illness based on its classical appearance on MRI and radiography.
Dr. Naveen Sathiyaseelan, Department of Orthopaedics, Saveetha Institute of Medical and Technical Sciences, Chennai, Tamil Nadu, India. E-mail: spnaveen17@gmail.com
Introduction: Ankylosing spondylitis (AS) is a chronic inflammatory disorder that primarily affects the spine and sacroiliac joints, leading to pain, stiffness, and progressive thoracolumbar kyphotic deformity. A key complication in advanced AS is the development of Andersson lesions (AL), degenerative vertebral lesions resulting from the disease’s progression. These lesions can cause significant mechanical pain, often mistaken for the chronic discomfort associated with AS. The exact cause of AL remains unclear, with hypotheses ranging from spinal stress fractures to delays in the ankylosing process. Understanding AL’s pathophysiology is essential for timely diagnosis and effective management.
Case Report: A 52-year-old male presented with a 20-year history of diffuse abdominal pain, later developing insidious lower back pain over the past 2 months. The pain was aggravated by walking and prolonged standing. Physical examination revealed tenderness in the D11 region of the spine, with limited chest expansion and positive findings on the modified Schober’s test. Radiographic studies showed irregularities and erosions at the D11-D12 vertebral levels, and magnetic resonance imaging confirmed the presence of an AL associated with asymmetrical bilateral sacroiliitis. The patient tested positive for human leukocyte antigen-B27, supporting a diagnosis of AS with an AL. Medical management, including methotrexate, sulfasalazine, non-steroidal anti-inflammatory drugs, and corticosteroids, led to significant pain reduction and improved mobility. The patient’s condition remained stable with continued treatment over a 2-year follow-up period.
Conclusion: AL s are chronic, often overlooked complications of AS that can lead to spinal instability and neurological deficits if untreated. Early recognition and management are critical to preventing progressive kyphotic deformities and associated complications. While conservative treatment remains the cornerstone for managing AL, surgical intervention may be required in cases of severe pain, deformity, or neurological involvement. Understanding AL’s presentation and treatment options is vital for improving patient outcomes in AS.
Keywords: Ankylosing spondylitis, Andersson lesion, spine, human leukocyte antigen B-27.
A chronic inflammatory illness that mostly affects the spine and sacroiliac joints, ankylosing spondylitis (AS) causes pain, stiffness, and a gradual thoracolumbar kyphotic deformity [1]. It is undoubtedly a debilitating disease [2]. It is referred to as a disco-vertebral lesion or degenerative vertebral lesion that appears as a late consequence for individuals with AS [3]. The expression “Andersson lesion” honors “Olof Andersson,” a Swedish radiologist who initially reported these results in 1937 while employed at Stockholm’s St. Eriks Hospital [4]. Commonly affected areas include the sacroiliac joint, the vertebrae in the lower back, tendon and ligament attachment sites (primarily in the spine and sometimes along the back of the heel), the intercostal cartilage, and hip and shoulder joints. The mobility of the affected joints is decreased and it might gradually worsen over time [5]. A fracture’s persistent instability and aseptic inflammation aided in the development of AL in AS [6]. Men are at higher risk than women [7]. In the advanced stages of the illness, the spine undergoes a gradual process of bone formation in the apophyseal joints, annulus fibrosis, flaval ligaments, anterior longitudinal ligament, and interspinous ligaments, leading to a fully fused spine, commonly known as a “bamboo spine.” Although the exact cause of AS is not known, genetic factors, particularly the presence of the human leukocyte antigen (HLA-B27) gene, are believed to play a significant role. Since its first description by Andersson in 1937, the understanding of AL, including its natural progression, underlying mechanisms, clinical presentation, diagnostic challenges, imaging characteristics, and even its terminology, has been subject to debate. One important fact that needs recognition is that there’s no proof that the source is infectious. A perusal of the literature revealed only two cases with an infected AL. Papaioannou et al. [8] have reported that the causative organism is Enterococcus species, and the same pathogen could also be recovered in urine cultures.
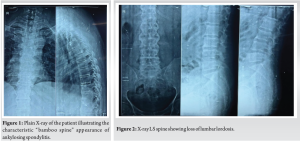
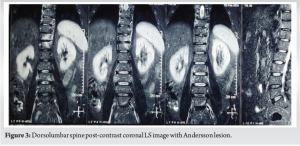
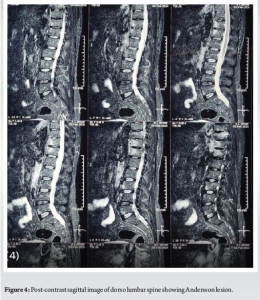
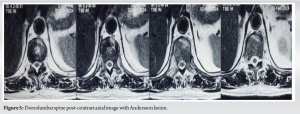
A 52-year-old man presented with a 20-year history of diffuse, non-radiating abdominal pain, localized to the right lower abdomen and aggravated by straining. He was diagnosed with a duodenal ulcer after an endoscopy and began treatment. He also reported 2 months of progressively worsening lower back pain, aggravated by walking and standing, without a history of trauma, tuberculosis, or constitutional symptoms. On examination, he had tenderness over the D11 region of the spine, with limited chest expansion and restricted, painful movements of the cervical and lumbar spine. Straight leg raise test and Faber’s tests were negative, with no motor or sensory deficits. HLA-B27 was positive, while the rheumatoid factor was negative. Radiographs showed loss of lumbar lordosis, with irregularities and erosions at the D11-D12 vertebral bodies (Fig. 1 and 2). Magnetic resonance imaging (MRI) revealed asymmetrical bilateral sacroiliitis, with paravertebral enhancement and syndesmophytes at D11-D12 and L2-L3. The Andersson lesion (AL) was hemispherical, hypointense on T1, and hyperintense on T2 and STIR images, with signs of reactive sclerosis and vertebral end plate erosions (Fig. 3-5). Right sacroiliitis with mild joint space reduction on the left side suggested chronic sacroiliitis. Based on these findings and the positive HLA-B27, a diagnosis of AS with AL was confirmed, with differential diagnoses including Pott’s spine, pyogenic infection, and malignancy.
The patient was started on the following medications: Methotrexate (7.5 mg/week, escalated to 15 mg/week for 2 weeks), folic acid (5 mg/week), oral non-steroidal anti-inflammatory drugs (NSAIDs) (naproxen 500 mg 2 times a day), sulfasalazine (1000 mg twice daily), and injectable methylprednisolone acetate (80 mg/week for 6 weeks). In addition, the patient was scheduled for D11-D12 debridement. Posterior stabilization and biopsy were offered but the patient was unwilling for the same due to financial constraints. After 6 weeks of treatment, the patient had major improvement in pain which was evaluated with Visual Analog Scale (VAS) score which had come down from a score of 8 at presentation to a score of 4. At 6 weeks, he also started walking without support. With a VAS score of 2 at the 2-year follow-up, the patient is responding well to treatment with methotrexate (22.5 mg/week) and sulfasalazine 1000 mg twice every day.
AL is a degenerative vertebral lesion, also known as a disco-vertebral lesion, that arises as a late consequence in patients with AS. It is an inflammatory involvement of the intervertebral discs caused by spondylarthritis (SpA). It is reported in about 8% of patients with AS and is typically triggered by minor trauma. As the disease progresses, inflammatory pain typically diminishes, and mechanical pain arising from AL becomes the initial symptom, often overlooked due to the chronic nature of AS. There is a continuing debate on the precise etiopathology of ALs; theories include spinal stress fractures and localized delays in the ankylosing process. The innate immune system is crucial in AS due to the uncontrolled activity of innate and innate-like immune cells in disease-prone areas. Because the intestine is the site of interaction between the intestinal microbiota and mucosal-associated immune cells, it plays a role in the symptoms of disease [9]. The pathophysiology of the articular appearance of AS may also involve biomechanical factors, such as entheseal micro-trauma. The pathophysiology of AS is linked to a number of factors, including immunity, environment, infection, and genetics [10]. According to one current view, AS is caused by the conformational plasticity of HLA-B27, the most important risk gene [11]. Research has shown that immune cells including T cells and B cells interact with bone cells like mesenchymal stem cells, osteoblasts as well as osteoclasts. These complicated relationships are also facilitated by a variety of cytokines and chemicals that are expressed by both the skeletal and immune systems. Recognizing the cellular and molecular processes that underlie the etiology of AS would establish a basis for investigating possible novel therapeutic approaches. The inflammatory illness known as AS begins in the sacroiliac joint and progresses to the point that the spine fuses to form the “bamboo spine.” It is part of the SpA disease category, which also includes inflammation of the tendons, ligaments, and peripheral joints in addition to the spine. The 2009 release of the Assessment of SpA International Society classification standards, which recognized axSpA as the single entity contrasted to peripheral SpA, is regarded as an event in the history of AS classification. “Radiographic axSpA (r-axSpA)” has essentially replaced the term “ankylosing spondylitis.” Radiographic features of AL s include irregularities and erosions of the vertebral endplates, which are considered late features of SpA. Clinicians should be alert to occult AS lesions, which can be undetectable on plain radiographs but can be identified by MRI or computed tomography (CT) scans. CT is superior for identifying posterior element defects, spinal stenosis, paravertebral soft-tissue swelling, and vacuum phenomenon in patients with AS who have developed pseudarthrosis. Diagnosing AS is challenging due to existing spinal modifications or pre-existing osteoporosis, and it shares radiographic similarities with infectious spondylodiscitis. Patients are sometimes started on anti-tuberculosis treatment as the lesions are misdiagnosed to be tubercular [12]. There is no consensus on using the modified New York criteria in diagnosing AS [13]. The general management of different types of SpA includes physical therapy, exercise, medicine, proper posture, and other measures including heating or cooling the affected area to reduce pain. Orthopedic surgery may potentially be a viable choice in extreme circumstances. In the case that AS is not well treated, the spine may develop fully ankylosing ligaments, tiny joints, and intervertebral discs, which would impair motor function and cause deformity and rigidity in the spine. Surgical management of AS is often necessary due to the aggravation of pain, deformity, or neurological damage. Surgical intervention involves instrumentation and fusion, with correction of any kyphotic deformity if present. Various surgical techniques, including decompression, instrumentation, bone grafting, and corrective osteotomies, have been reported for managing deformities associated with AL. Medical treatment options for AS involve NSAIDs, physical therapy and exercise, lifestyle modifications, and the bath ankylosing spondylitis disease activity index (BASDAI). The BASDAI helps assess the efficiency of existing drug therapy, determine the need for new drug therapy, and identify patients suitable for clinical trials evaluating new AS drug therapies. ALs, which are irregularities and erosions of vertebral endplates, are considered late features of SpA. However, clinicians should be alert to occult AS lesions, including AL, which are undetectable on plain radiographs but can be identified by CT and/or MRI. Delaying the diagnosis and treatment of ALs might exacerbate patients’ worsening pain, kyphotic deformity, and neurological impairments. As individuals with AS develop pseudoarthrosis, CT is better at detecting posterior element abnormalities, vacuum phenomena, spinal stenosis, and paravertebral soft-tissue swelling. MRI provides a better view of spondylodiscitis, as early edematous changes are not visible on conventional radiographs. AL lesions are chronic, and their diagnosis and management are crucial for managing the condition.
It is crucial to identify Anderson lesions, as they are chronic lytic lesions that are overlooked due to chronic pain of AS, which leads to instability of the spine. The foundation of treatment for progressive symptoms, severe discomfort, kyphotic deformity, or neurological problems is conservative management. Surgical management is required to alleviate pain, neurological Involvement, and its progression in progressive kyphotic deformities.
Monitoring and early treatment are most successful when initiated before irreversible damage occurs. Regular follow-up with a rheumatologist is essential.
References
- 1.Von Bechterew W. Steifigkeit der wirbelsäule und ihre Verkrümmung als besondere Erkrankungsform. Neurol Zentralblatt 1983;12:426-34. [Google Scholar | PubMed]
- 2.McGonagle D, Lyn Tan A, Wakefield R, Emery P. Imaging in ankylosing spondylitis. In: Van Royen BJ, Dijkmans B, editors. Ankylosing Spondylitis-diagnosis and Management. New York: Taylor & Francis Group; 2006. p. 71-81. [Google Scholar | PubMed]
- 3.Andersson O. Röntgenbilden vid spondylarthritis ankylopoetica. Nord Med Tidskr 1937;14:2000-2. [Google Scholar | PubMed]
- 4.Wu X, Lu H, Xu H. Andersson lesion in ankylosing spondylitis. Rheumatol Immunol Res 2022;3:45. [Google Scholar | PubMed]
- 5.Bai LL, Du JP, Xue XK, Hao DJ, Wang WT. The CT image changes in ankylosing spondylitis from fracture to Andersson lesions: A case report and literature review. Clin Interv Aging 2020;15:2227-30. [Google Scholar | PubMed]
- 6.Dave BR, Ram H, Krishnan A. Andersson lesion: Are we misdiagnosing it? A retrospective study of clinic-radiological features and outcomes of short segment fixation. Eur Spine J 2011;20:1503-9. [Google Scholar | PubMed]
- 7.Pai SN, Kailash K, Vignesh J, Sudhir G. Andersson lesion in ankylosing spondylitis. BMJ Case Rep 2022;15:e248542. [Google Scholar | PubMed]
- 8.Papaioannou I, Pantazidou G, Repantis T, Baikousis A, Korovessis P. An infected Andersson lesion presented with incomplete paraplegia in a patient with ankylosing spondylitis. A unique case report with literature review. Spinal Cord Ser Cases 2022;8:73. [Google Scholar | PubMed]
- 9.Zhang H, Hu J, Zhang C, Gao M, Zhao H. Treating thoracic-lumbar Andersson lesion in patients with ankylosing spondylitis: Case series. Ann Med Surg (Lond) 2023;85:1420-4. [Google Scholar | PubMed]
- 10.Liu L, Yuan Y, Zhang S, Xu J, Zou J. Osteoimmunological insights into the pathogenesis of ankylosing spondylitis. J Cell Physiol 2021;236:6090-100. [Google Scholar | PubMed]
- 11.Mauro D, Thomas R, Guggino G, Lories R, Brown MA, Ciccia F. Ankylosing spondylitis: An autoimmune or autoinflammatory disease? Nat Rev Rheumatol 2021;17:387-404. [Google Scholar | PubMed]
- 12.Chan FL, Ho EK, Chau EM. Spinal pseudarthrosis complicating ankylosing spondylitis: Comparison of CT and conventional tomography. AJR Am J Roentgenol 1988;150:611-4. [Google Scholar | PubMed]
- 13.Kim SK, Shin K, Song Y, Lee S, Kim TH. Andersson lesions of whole spine magnetic resonance imaging compared with plain radiography in ankylosing spondylitis. Rheumatol Int 2016;36:1663-70. [Google Scholar | PubMed]