Giant cell tumor with chondroid differentiation, being a rare histological variant, is diagnosed with the help of IHC and managed as a conventional GCT as the clinical behavior of both is similar.
Dr. Ashish Gulia, Department of Bone and Soft Tissue, Homi Bhabha Cancer Hospital and Research Centre, New Chandigarh, Punjab, India. E-mail: aashishgulia@gmail.com
Introduction: Giant cell tumor (GCT) with chondroid differentiation is an uncommon entity. We report a case report of a 44-year-old female presenting with GCT of the left distal femur showing chondroid differentiation.
Case Report: A 44-year-old female presented to us with complaints of pain and swelling in the left distal femur for 4 months. On further evaluation, X-ray revealed an osteolytic lesion in the distal femur with ill-defined margins and a narrow zone of transition. Magnetic resonance imaging showed a well-defined lesion in epi-metaphyseal lesion measuring 64 × 52 × 38 mm on the left side of distal femur, associated with a large soft tissue mass. Biopsy was done which revealed several fragments of cartilage with only a few fragments showing oval-to-spindle cell proliferation with areas of stromal hyalinization with some tumor fragments showing no unequivocal substantial number of osteoclast-like giant cells. Immunohistochemistry examination showed diffuse and strong nuclear positivity for H3.3G34W. Considering the diagnosis of GCT, the patient underwent curettage and cementing and plating. Final histopathology was also consistent with GCT, showing chondroid differentiation. The patient is 18-month post-surgery and is doing fine without any recurrence.
Conclusion: This report details the radiological and pathological findings of the GCT of distal femur exhibiting cartilage matrix. It behaves as a GCT without cartilage matrix and should be treated accordingly. Furthermore, immunohistochemical staining with antibodies against the mutant-specific H3.3 protein or identification of H3.3G34A guides us for confirmation of diagnosis.
Keywords: Giant cell tumor, chondroid, immunohistochemistry.
Giant cell tumor (GCT) of bone is a rare, typically benign, yet locally aggressive neoplasm that commonly arises in the epiphysis of long bones, particularly in young adults. While GCTs are generally well-characterized, the occurrence of chondroid differentiation within these tumors or GCT in an existing chondroid lesion is an exceptionally rare phenomenon [1]. This unusual feature presents a significant diagnostic challenge due to its atypical histopathological presentation and potential implications for clinical management. We present a case report of a 44-year-old female with a rare instance of GCT with extensive chondroid differentiation, discussing its diagnostic complexities and therapeutic considerations.
The patient is a 44-year-old female who presented with a progressively enlarging, painful swelling in the left distal femur. The swelling had been present for several months and was associated with localized pain that worsened over time. Radiographic imaging (Fig. 1) revealed an osteolytic lesion in the distal femur with ill-defined margins and a narrow zone of transition, suggestive of a GCT (Campanacci grade 2). Magnetic resonance imaging (MRI) of the lesion (Fig. 2) was done which revealed a well-defined lesion in epi-metaphyseal lesion measuring 64 × 52 × 38 mm on the left side of distal femur, associated with a large soft tissue mass, with no intra-articular extension.
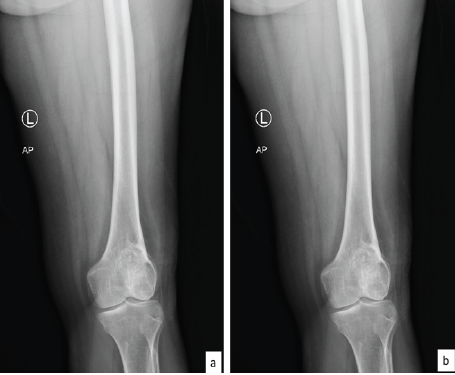
Figure 1: Pre-operative X-rays showing lesion in left distal femur; (a) anteroposterior and (b) lateral view.
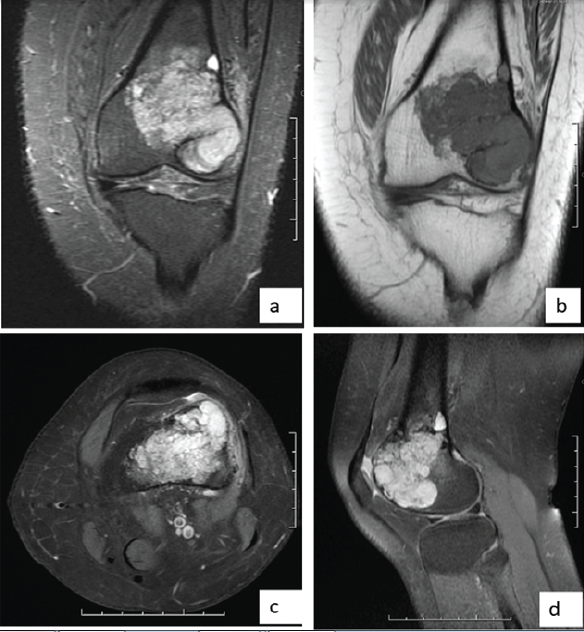
Figure 2: Magnetic resonance images. (a) T2w coronal image, (b) T1w coronal image, (c) T2w axial image, (d) T2w sagittal image showing lesion in the distal aspect of left femur.
This was followed by biopsy of the lesion which revealed several fragments of cartilage with only a few fragments showing oval-to-spindle cell proliferation with areas of stromal hyalinization. No unequivocal substantial number of osteoclast-like giant cells was seen in the few tumor fragments. The tumor cells showed diffuse and strong nuclear positivity for H3.3G34W immunohistochemistry and only patchy weak nuclear positivity for SATB2 and were negative for CD99, CD34, STAT6, and S100. A diagnosis of GCT with unusual chondroid metaplasia was rendered (Fig. 3a, b, c, d, e). This case was discussed in the multidisciplinary meeting and it was decided to proceed with curettage with cementing and plating (Fig. 4) for this patient. Intraoperatively, frozen section was sent, which showed giant cells along with chondroid differentiation; this chondroid component was benign. Final histopathology revealed similar findings along with areas with characteristic histopathological findings of GCT of bone in the form of proliferation of mononuclear stromal cells and evenly dispersed numerous osteoclast-like giant cells. Mitosis was infrequent, and no atypical mitotic figure was noted. No area of necrosis was identified. The cartilaginous component was composed of well-differentiated hyaline cartilage with basophilic matrix. There was no increase in cellularity or nuclear atypia. The immunohistochemistry for H3.3G34W was diffusely and strongly positive in the stromal component of the tumor, whereas it was negative in the osteoclast-like giant cells and the cartilage matrix (Fig. 3f, g, h). The patient was kept under observation. This patient has been on follow-up 18-month post-surgery and is doing fine without any symptoms, with a knee range of motion from 0 to 110°.
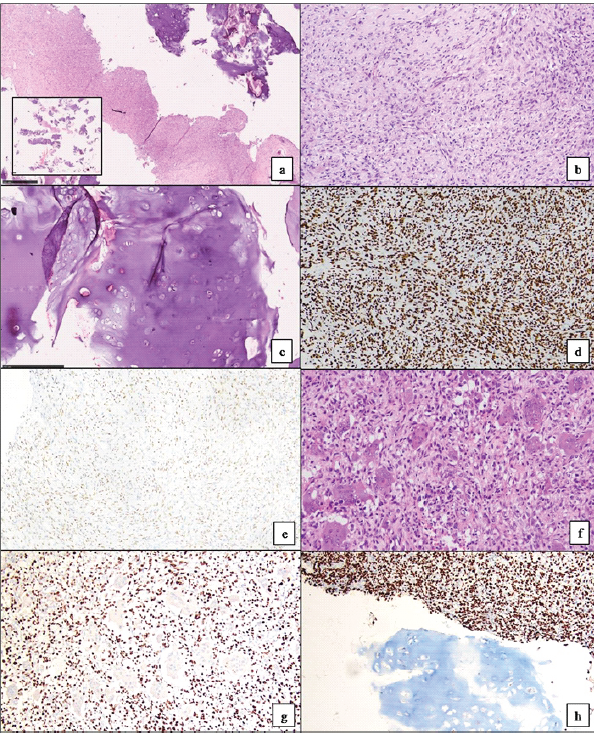
Figure 3: Microscopic pictures from the biopsy showed dominantly hyaline cartilage (3a inset, H&E stain, scanner view) with a few fragments showing proliferation of oval to spindle-shaped tumor cells (3a, H&E stain, ×40). There was background stromal hyalinization and lack of osteoclastic giant cells (3b, H&E stain, ×200). The cartilage component showed basophilic matrix with no increase in cellularity or any nuclear atypia (3c, H&E stain, ×100). By Immunohistochemistry, tumor cells showed diffuse and strong positivity for H3.3G34W (3d, ×100) and weak nuclear positivity for SATB2 (3e, ×100). Microscopic sections from the curettage specimen showed similar features along with characteristic morphology of giant cell tumor of bone (3f, H&E stain, ×200). H3.3G34W immunohistochemistry was negative in the osteoclastic giant cells (3g, ×100) and the cartilage matrix (3h, ×100).
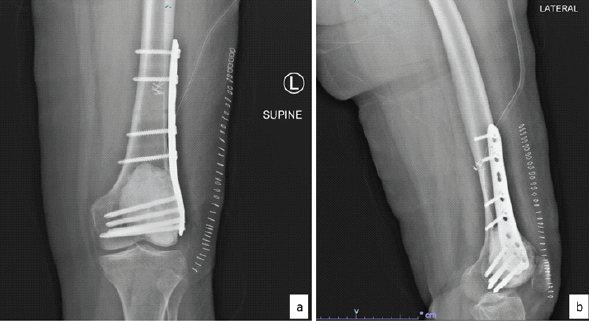
Figure 4: Post-operative X-rays. (a) Anteroposterior and (b) lateral view.
GCT of bone is a rare, locally aggressive, and typically benign bone neoplasm which is lytic, eccentric subchondral lesion that mostly affects the epiphyseal and metaphyseal regions of long bones, particularly around the knee joint, in young adults. The presence of a sclerotic rim and a calcified matrix with an open epiphysis are features which can be used to differentiate cartilage-forming tumor’s, such as chondroblastomas, from GCTs of the bone [2]. The characteristic histological appearance of GCT displays a high number of osteoclast-like multinucleated giant cells, with a background population of plump, epithelioid to spindle-shaped mononuclear cells [3]. The tumor is characterized by mutation in the H3F3A gene, most often G34W/G35W and less frequently G34L, G34V/G35V, G34R, G34M/G35M, and G35E. Immunohistochemically, the neoplastic tumor cells are usually extensively and strongly positive for H3.3, p63, and SATB2 and are negative for S100. The commonly mutated protein product H3.3 is highly specific for GCT and helps in distinguishing the same from other benign giant cell-rich lesions [4]. To the best of our knowledge, very few cases of GCT with chondroid differentiation have been published in the literature. In 2005, Do et al. published a case report of a 31-year-old male with lesion involving the acetabulum and adjacent pubis diagnosed as chondroblastoma on biopsy and classic GCT with foci of cartilage matrix on final histopathology [5]. Al-Ibraheemi et al. [6] posted a series on the histologic spectrum of GCT of bone, of 63 patients, where 2 cases showed foci of hyaline-like cartilage. Recently, in 2020, a case series of 17 cases was published showing GCT of bone with cartilage matrix. This study had 7 males and 10 females with proximal fibula and femur being the most commonly involved sites. Immunohistochemistry was performed on 11 cases and the neoplastic cells in the classic GCT areas were strongly positive for H3.3 (9/9 cases, 100%), p63 (5/5 cases, 100%), and SATB2 (4/4 cases, 100%) and negative for S100 (9/10 cases, 90.0%) and SOX9 (7/8 cases, 87.5%) [7]. Our case involved the distal femur with histopathology showing several fragments of cartilage along with tumor fragments showing mononuclear stromal cells and many dispersed osteoclast-like giant cells. Our case showed well-differentiated hyaline cartilage with basophilic matrix which was noted in 3 of the 17 cases of the case series by Brcic et al. [7]. The stromal cells showed diffuse and strong positivity for H3.3G34W and only patchy weak nuclear positivity for SATB2 and were negative for CD99, CD34, STAT6, and S100. The cartilage matrix in our case did not show any positivity for H3.3G34W immunohistochemistry which is in contrast to the finding of the study of Brcic et al. [7]. In that study, IHC for H3.3 was performed in 9 cases and all of them showed positivity in the cartilage matrix along with stromal cells. This case report is limited by being a single-patient study with a short follow-up period, which restricts the ability to generalize findings, assess long-term outcomes, or capture recurrence. Due to the rarity of GCT with chondroid differentiation, broader conclusions regarding prognosis or optimal management cannot be drawn.
This report details the radiological and pathological findings of the GCT of the distal femur exhibiting cartilage matrix. It behaves as a giant cell tumor without cartilage matrix and should be treated accordingly. Furthermore, immunohistochemical staining with antibodies against the mutant-specific H3.3 protein or identification of H3.3G34A guides us for confirmation of diagnosis.
1. GCT with chondroid differentiation is a rare histological variant which can mimic other cartilage-forming tumors and poses a significant diagnostic challenge.
2. Immunohistochemistry, especially H3.3G34W positivity in stromal cells, is crucial for confirming GCT and distinguishing it from chondroblastoma or chondrosarcoma.
3. The presence of cartilage matrix does not alter the clinical behavior of the tumor; such cases should be treated similarly to conventional GCTs.
4. Awareness of this variant is essential in multidisciplinary settings to avoid misdiagnosis and guide appropriate surgical management.
References
- 1. Athanasou NA, Bansal M, Forsyth RP, Sapi Z. Giant cell tumour of bone. In: Fletcher CD, Bridge JA, Hogendoorn PC, Mertens F, editors. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. Lyon, France: International Agency for Research on Cancer; 2013. p. 321-4. [Google Scholar] [PubMed]
- 2. Stacy GS, Peabody TD, Dixon LB. Mimics on radiography of giant cell tumor of bone. AJR Am J Roentgenol 2003;181:1583-9. [Google Scholar] [PubMed]
- 3. Fechner RE. Tumors of the bone and joints. Atlas Tumor Pathol 1993;8:266-71. [Google Scholar] [PubMed]
- 4. Yamamoto H, Iwasaki T, Yamada Y, Matsumoto Y, Otsuka H, Yoshimoto M, et al. Diagnostic utility of histone H3.3 G34W, G34R, and G34V mutant-specific antibodies for giant cell tumors of bone. Hum Pathol 2018;73:41-50. [Google Scholar] [PubMed]
- 5. Do I, Ryu KN, Han CS, Park YK. Giant cell tumor with an unusual cartilage matrix. Korean J Pathol 2005;39:269-72. [Google Scholar] [PubMed]
- 6. Al-Ibraheemi A, Inwards CY, Zreik RT, Wenger DE, Jenkins SM, Carter JM, et al. Histologic spectrum of giant cell tumor (GCT) of bone in patients 18 years of age and below: A study of 63 patients. Am J Surg Pathol 2016;40:1702-12. [Google Scholar] [PubMed]
- 7. Brcic I, Yamani F, Inwards CY, Sumathi V, Dodd L, Kreiger PA, et al. Giant cell tumor of bone with cartilage matrix: A clinicopathologic study of 17 Cases. Am J Surg Pathol 2020;44:748-56. [Google Scholar] [PubMed]










