Ewing’s–like adamantinoma is a rare entity requiring clinical, pathological and radiological concurrence to arrive at the diagnosis. Ilizarov bone transport with neo-adjuvant chemotherapy is a feasible limb salvage option.
Dr. Puneeth K Pai,
Department of Orthopedics,
Government Medical College, Kozhikode, Kerala, India.
E-mail: puneethpaik@gmail.com
Introduction: Adamantinoma is a rare low-grade malignant tumor in young adults. Recent advances in molecular techniques have shown different variants with discordance between genotype and phenotype. This poses a challenge in diagnosis and management. Ewing’s-like adamantinoma is one such variant. The appropriate treatment protocol for this dedifferentiated tumor remains to be established. Here, we present a rare case of Ewing’s-like Adamantinoma treated with limb salvage surgery using three step Ilizarov technique with good results.
Case Report: A 38-year-old lady presented at our OPD complaining of recent onset pain and gradual increase in size over a long standing swelling in the right lower tibia. Radiologically was diagnosed as Ewing’s but was not responsive to chemotherapy. A Tru-Cut biopsy showed histological picture of dedifferentiated adamantinoma. Immunohistochemistry showed CD99 positivity. FISH revealed (11;22) translocation confirming Ewing’s-like adamantinoma. She was managed with wide excision followed by staged Ilizarov distraction-osteogenesis and bone transport to correct the 13 cm discrepancy in bone length. There have been no signs of recurrence on post-resection follow-up of 2 years. Patient attained full range of knee and ankle movements.
Conclusion: The controversy as to what constitutes the difference between adamantinoma-like Ewing’s and Ewing’s-like adamantinoma persists despite technological advances. The appropriate treatment protocol remains to be established. Ilizarov three step techniques provide a feasible alternative to amputation while circumventing the difficulties of large bone reconstruction in the distal tibia.
Keywords: Adamantinoma, Ewing’s-like, Ilizarov, bone transport, dedifferentiated, malignant bone tumor.
Adamantinoma is a primary low-grade, malignant bone tumor of epithelial origin, [1]. Although predominantly localized to the mid-tibial diaphysis, cases of synchronous or isolated lesions in fibula have been reported. It is a rare neoplasm, comprising only 0.1–0.5% of all primary bone tumors [2]. Ewing’s sarcoma is a highly malignant skeletal tumor, mainly affecting children. It was originally characterized by James Ewing in 1921 as having uniform, densely packed small cells, without distinct cytoplasmic borders or prominent nucleoli [3,4]. It is now considered to be of neuroectodermal origin. The differential diagnosis is of small round blue cell tumors which include rhabdomyosarcoma, neuroblastoma, and lymphoma [4]. Over 90% of Ewing’s sarcomas are genetically characterized by chromosomal translocation of t(11;22) (q24;q21) [1], resulting in fusion of the EWS/FLI-1 gene [5]. In the past, genotypic and phenotypic overlapping of Ewing sarcoma and adamantinoma have been noted. It has been brought into better focus with advancement of immunohistochemistry and genetic analysis. Ewing’s-like adamantinoma is a rare variant with clinical presentation, radiology, and histology of adamantinoma but with genotype suggestive of Ewing’s sarcoma. The focus of this case report is to draw attention to an extremely rare variant of adamantinoma and the outcome of our treatment method. There are no case reports as per our literature review where Ewing’s variant of adamantinoma has been treated by limb salvage surgery using Ilizarov bone transport. Here, we report a case of tibial adamantinoma with Ewing’s genotype in a 38-year-old lady treated with a three step limb salvage approach.
A 38-year-old lady presented at our OPD complaining of swelling in the right lower leg, incidentally noticed 8 years ago, gradually increasing in size over the past 6 months. It was associated with pain on walking for more than 10 m. On examination a 15 cm × 6 cm × 3 cm hard swelling continuous with the anterior border of right tibia was noted. There was no local rise of temperature or tenderness. The overlying skin was pinchable. Plain radiograph showed an eccentric, expansile, lytic lesion in the distal third of right tibia with scalloping, and antero-lateral cortical break (Fig. 1, 2, 3).

MRI showed irregular eccentric expansile lesion measuring 6.5 × 2.2 × 5.8 cm in the anterior cortex of tibia with a narrow zone of transition. Extension into intramuscular and subcutaneous planes was noted. A separate lesion was noticed in the lower third of right fibular diaphysis measuring 7 × 2 mm (Fig. 2, 3).

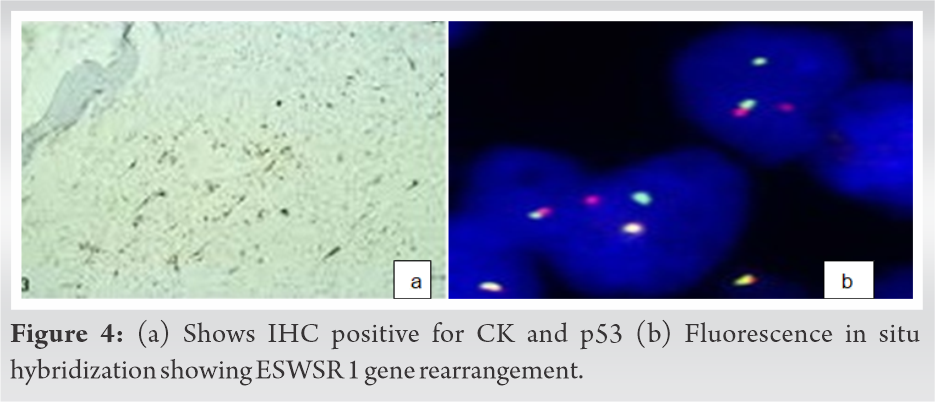
A Tru-cut biopsy of the lesion showed high grade spindle cell neoplasm suggestive of adamantinoma with areas of round blue cell rests. There were areas of sarcomatous changes consistent with dedifferentiation. Immunohistochemistry was positive for Ki 67-moderate (30–40%), cytokeratin, p53, and CD99. Cytogenetic analysis showed Chromosome (11;22) translocation suggestive of Ewing’s-like adamantinoma. Fluorescent in situ hybridization (FISH) performed on formalin fixed paraffin embedded tissue showed 22q12 (EWSR1) gene rearrangement in 32% of assessed cells confirming t(11;22) (q23;q12). After a clinico-patho-radiological correlation, a diagnosis of dedifferentiated adamantinoma variant, that is, Ewing’s-like adamantinoma was confirmed (Fig. 4, 5, 6).
Neo-adjuvant chemotherapy was administered – two cycles of Vincristine, Doxorubicin, and Cyclophosphamide and two cycles of Ifosfamide and Etoposide. On post-chemotherapy day 14, she underwent wide excision of right distal and middle tibia. The extent of resection was planned according to the preoperative MRI and in consultation with the surgical oncologist. About 13 cm of bone was excised followed by stabilization with Ilizarov ring fixator and cement spacer as the first step. The purpose of cement spacer was to prevent contracture of soft tissue which might hinder future bone transport, eliminate the dead space thereby reducing risk of infection and also maintain limb length. The completeness of the resection was confirmed by post-operative histopathological examination of the whole specimen which showed clear margins. Resected tumor margins had a clearance of 3 cm proximally and 2 cm distally.

The second step performed after three months involved removal of cement spacer and corticotomy between proximal and middle rings. Bone transport was done over a period of 6 months. The third step included docking of the transported fragment into the distal segment after freshening bone margins and additional corticocancellous bone grafting from ipsilateral iliac crest. Early mobilization was done with partial weight bearing and exercise regimen for knee, ankle, and toes to prevent muscle wasting and contracture. Thus, the 13 cm discrepancy in limb length was corrected. There have been no signs of recurrence on post-resection follow-up of 2 years (Fig. 6).
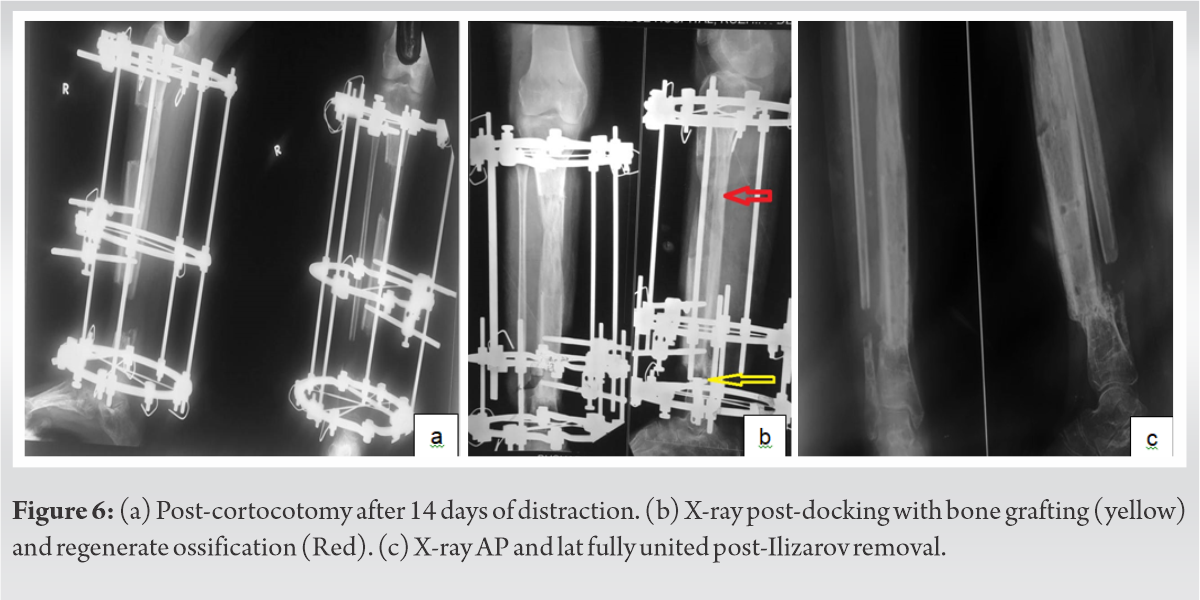
After docking of the fracture site, patient developed surgical site infection which was treated with antibiotics. Full range of movement of knee and ankle was attained. Union of the docking site was confirmed clinically and radiologically at 1 year, after which the ring fixator was removed. A cast brace was applied and patient was started on unassisted weight bearing.
Adamantinoma is a low-grade malignancy composed of epithelial cells in a fibrous or osteofibrous stroma. The first reported example of adamantinoma is attributed to Maier in 1900 [6]. In 1913, Fisher [7] named the lesion “primary adamantinoma of the tibia” because of its striking histologic resemblance to the jaw “adamantinoma” (ameloblastoma). These tumors have been also referred to as “malignant tumor of humerus with features of adamantinoma and Ewing’s sarcoma” by Meister et al. [8], Ewing-like adamantinoma [9], juxta-cortical adamantinoma (simulating Ewing tumor) by Ishida et al. [10,11], and “adamantinoma of the pretibial soft tissue” by Mills and Rosai [12]. A similar tumor was recently reported by Schofield et al [13]. Adamantinoma occurs in the second to third decades. The median patient age is 25–35 years. It is more common in men than women, with a ratio of 5:4 [14]. It is rare in children [15]. The tumor has a predilection for the long bones and specifically, the tibia (80–85% of cases). In 10–15%, the lesion is also found in the ipsilateral fibula [16]. In our case too, there was predominant involvement of tibia along with involvement fibula. There are reports of all other major limb bones having been involved [17]. Other bones that are involved, in order of decreasing frequency are humerus, ulna, femur, fibula, radius, innominate bone, ribs, spine, and rarely small bones of the hand and foot [18,19,20,21,22,23,24,25]. Rarely, adamantinoma can exclusively arise from the pretibial soft tissues without bony involvement [12,26]. The initial symptoms of adamantinoma are swelling and pain with severity depending on location and extent of the disease. The onset is insidious and its course shows a slow, progressive character. The patient often tolerates symptoms for many years before seeking medical attention. A history of significant trauma has been noted in about 60% of 200 cases reviewed by Moon and Mori [14]. Most patients present with swelling with or without pain. In our case, the swelling was asymptomatic for 8 years before the development of pain, accompanied by rapid progression of size of the swelling. Patient may present with deformity of the tibia due to involvement of anterior tibial surface, probably due to cortical break. Pathological fracture may be present in as many as 23% of the patients [27]. There have been two case reports of paraneoplastic hypercalcemia associated with tibial adamantinoma and pulmonary metastasis [28,29]. Spinal lesions may manifest as neurologic symptoms in addition to pain [24]. Adamantinoma can be classified into classical and de-differentiated. De-differentiated varieties include Ewing’s like adamantinoma, adamantinoma-like Ewing’s, and osteofibrous dysplasia.
Ewing’s-like adamantinoma is the least common variant of adamantinoma. There is no clinical difference in the presentation or progression between the classical and dedifferentiated variants. The variant is characterized by anastomosing cords of small, uniform, round cells set in a myxoid stroma. These cells exhibit features of both epithelial cells and neuroendocrine cells on ultrastructural examination. On the contrary, the histopathology in our case showed high grade spindle cell like neoplasm, making it a phenotypic variant. Immunohistochemical studies have shown the tumor cells to contain both epithelial and neural antigens including the Ewing’s sarcoma-related antigen [8,9]. Immunohistochemistry in this case showed cytokeratin, p53 and moderate (30-40%) Ki-67 positivity. CD99 was positive. Cytogenetic analysis showed (11;22) translocation suggestive of Ewing’s-like adamantinoma. FISH performed on formalin fixed paraffin gene embedded tissue showed 22q12 (ESWSR1) gene rearrangement in 32% of assessed cells confirming t(11;22)(q23;q12). These findings are in coherence with reports by Meister et al. and Lipper et al. [8,9]. On the contrary Bridge et al. [30] documented (11;22) translocation in the nuclei of cytokeratin-immunoreactive cells, and therefore, considered the tumors to be variants of Ewing’s sarcoma rather than Ewing’s-like adamantinoma. They termed the lesion “adamantinoma-like Ewing’s’’. Hauben et al, however, reported 12 cases of adamantinoma which lacked both EWS-FLI-1 and EWS-ERG fusion transcripts [31]. Folpe et al. described three cases of “adamantinoma-like” Ewing’s sarcoma that showed a distinctly nested, epithelioid growth pattern with surrounding desmoplasia, and characteristically expressed high molecular-weight cytokeratins and pan-cytokeratins with genetic confirmation [32]. Fujii et al. reported a case of adamantinoma-like Ewing’s sarcoma with EWS-FLI1 fusion gene [33]. Recently, the risk-adapted treatment strategy including high dose chemotherapy (HDCT) with peripheral blood stem cells transplantation (PBSCT) has been proposed [34]. However, some studies have demonstrated that HDCT with PBSCT in the treatment of Ewing’s sarcoma have limited benefits [35,36]. Further studies are needed to clarify the differences in prognosis and chemo-sensitivity between conventional adamantinoma, Ewing’s sarcoma, and “Ewing’s like adamantinoma.”
Reconstruction after wide resection in such tumors is difficult, primarily because of relative decreased vascularity of the distal tibia and its anatomical proximity to the neurovascular bundle and tendons. Thus, limb amputation would optimize postoperative function and minimize tumor recurrence and metastasis [37,38]. Addition of neoadjuvant chemotherapy reduces resection margins, while preserving the limb. Methods of major reconstruction after wide resection of malignant bone include allografts, treated-bone autografts, vascularized fibula grafts, and endoprosthetic replacement. Allografts can be physiologically reconstructed, resulting in good postoperative results, but adjuvant treatment increases complications [39]. Use of allografts in India is difficult because of lack of established bone banking system. Treated-bone autografts have been associated with good prognosis and post-operative function, but at the cost of bone strength. Vascularized fibula grafts result in bone fusion at an early stage. Furthermore, these grafts can be covered by soft tissue after wide resection, with good post-operative limb function. A major drawback is that the fibula is thinner than the distal tibia, increasing the risk of fracture of transplanted bone. In addition, this surgical procedure is complicated with the need for separate micro-vascular surgery team with long operation time [40,41]. Endoprosthetic replacement gives instant stability and quick recovery of ankle function, allowing load bearing to start early. However, late instability, misalignment, and difficulty securing the prosthesis to the talus have been associated with a high failure rate. Other reported complications include infections, inadequate soft-tissue coverage, and instability [42,43]. Ilizarov method has been widely employed in a variety of treatments for limb deformity, bone defect, and limb shortening [43]. In 1998, Ozaki et al. adopted Ilizarov method for bone transport in limb-salvage treatment following bone tumor resection for Ewing’s sarcoma. However, the incidence of complications (such as pin tract infection, delayed consolidation of regenerate, kinking of the skin, docking site nonunion, refracture) was high and bone formation was poor and slow. Multiple operations were conducted after initial surgery and the survival time of most patients was relatively short [44]. The complications considerably reduce the limb-salvage effect of Ilizarov method for bone transport, which may be attributed to the technical limitations and inadequate doses of chemotherapy.
There is considerable overlap of morphological and immunohistochemical features in adamantinoma and its variants, which due to technological advancement, can be readily distinguished with molecular and cytogenetic techniques. There still remains the controversy as to what constitutes the difference between adamantinoma-like Ewing’s and Ewing’s-like adamantinoma. Furthermore, appropriate treatment protocol of these dedifferentiated variants has not yet been established. Various treatment modalities including neo-adjuvant chemotherapy, wide-excision with bone defect reconstruction, and even amputation have been suggested.
Ewing’s like adamantinoma is a rare entity and requires clinico-radio-pathological correlation for diagnosis. Management protocols for these lesser known variants are not well established as there is still controversy regarding efficacy of chemotherapy in these cases. Use of Ilizarov bone transport is a viable alternative to amputation if wide excision with clear margins can be achieved.
References
- 1.Aurias A, Rimbaut C, Buffe D, Dubousset J, Mazabraud A. Translocation of chromosome 22 in Ewing’s sarcoma. C R Seances Acad Sci III 1983;296:1105-7. [Google Scholar | PubMed]
- 2.Ewing J. Classics in oncology. Diffuse endothelioma of bone. James Ewing. Proceedings of the New York pathological society, 1921. CA Cancer J Clin 1972;22:95-8. [Google Scholar | PubMed]
- 3.Turc-Carel C, Philip I, Berger MP, Philip T, Lenoir GM. Chromosome study of Ewing’s sarcoma (ES) cell lines. Consistency of a reciprocal translocation t(11;12)(q24;q12). Cancer Genet Cytogenet 1984;12:1-19. [Google Scholar | PubMed]
- 4.Ushigome S, Machinami R, Sorensen PH. Ewing sarcoma/ primitive neuroectodermal tumor (PNET). In: Fletcher CD, Unni KK, Mertens F, editors. WHO Classification of Tumors. Pathology and Genetics of Tumors of Soft Tissue and Bone. Lyon, France: International Agency for Research on Cancer; 2002. p. 153-4. [Google Scholar | PubMed]
- 5.Zucman J, Delattre O, Desmanze C, Plougastel B, Joubert I, Melot T, et al. Cloning a characterization of the Ewing’s sarcoma and peripheral neuroepithelioma t(11;22) translocation breakpoints. Genes Chromosomes Cancer 1992;5:271-7. [Google Scholar | PubMed]
- 6.Maier C. Ein primäres myelogenes Plattenepithelcarcinom der Ulna. Bruns Beitr Klin Chir 1900;26:533. [Google Scholar | PubMed]
- 7.Fisher B. Primary adamantinoma of the tibia. Z Pathol 1913;12:422-41. [Google Scholar | PubMed]
- 8.Meister P, Konrad E, Hubner G. Malignant tumor of humerus with features of ‘‘adamantinoma’’ and Ewing’s sarcoma. Pathol Res Pract 1979;166:112-22. [Google Scholar | PubMed]
- 9.Lipper S, Kahn LB. Case report 235. Ewing-Like adamantinoma of the left radial head and neck. Skeletal Radiol 1983;10:61-6. [Google Scholar | PubMed]
- 10.Ishida T, Kikuchi F, Oka T, Machinami R, Kojima T, Iijima T, et al. Case report 727: Juxtacortical adamantinoma of humerus (simulating Ewing tumor). Skeletal Radiol. 1992;21:205-9. [Google Scholar | PubMed]
- 11.Ishida T, Iijima T, Tikuchi F, Kitagawa T, Tanida T, Imamura T, et al. A clinicopathological and immunohistochemical study of osteofibrous dysplasia, differentiated adamantinoma, and adamantinoma of long bones. Skeletal Radiol. 1992;21:493-502. [Google Scholar | PubMed]
- 12.Mills SE, Rosai J. Adamantinoma of pretibial soft tissue. Clinicopathologic features, differential diagnosis, and possible relationship to intraosseous disease. Am J Clin Pathol 1985;83:108-14. [Google Scholar | PubMed]
- 13.Schofield DE, Conrad EU, Liddell RM, Yunis EJ. An unusual round cell tumor of the tibia with granular cells. Am J Surg Pathol 1995;19:596-603. [Google Scholar | PubMed]
- 14.Moon NF, Mori H. Adamantinoma of the appendicular skeleton updated. Clin Orthop 1986;204:215. [Google Scholar | PubMed]
- 15.Kumar D, Mulligan ME, Levine AM, Dorfman HD. Classic adamantinoma in a 3-year-old. Skeletal Radiol 1998;27:406-9. [Google Scholar | PubMed]
- 16.Keeney GL, Unni KK, Beabout JW, Pritchard DJ. Adamantinoma of long bones. A clinicopathologic study of 85 cases. Cancer 1989;64:730. [Google Scholar | PubMed]
- 17.Ulmar B, Delling G, Werner M, Huch K, Reichel H. Classical and atypical location of adamantinomas presentation of two cases. Onkologie 2006;29:276-8. [Google Scholar | PubMed]
- 18.Huvos AG, Marcove RC: Adamantinoma of long bones. A clinicopathological study of fourteen cases with vascular origin suggested. J Bone Joint Surg Am 1975;57:148-54. [Google Scholar | PubMed]
- 19.Sherman GM, Damron TA, Yang Y. CD99 positive adamantinoma of the ulna with ipsilateral discrete osteofibrous dysplasia. Clin Orthop Relat Res 2003;408:256-61. [Google Scholar | PubMed]
- 20.Makhson AN, Bulycheva IV, Kuz’min IV, Pavlenko TV. Adamantinoma of long tubal bones. Arkh Patol 2006;68:14-8. [Google Scholar | PubMed]
- 21.Bourne MH, Wood MB, Shives TC. Adamantinoma of the radius: A case report. Orthopedics 1988;11:1565-6. [Google Scholar | PubMed]
- 22.Lasda NA, Hughes EC Jr. Adamantinoma of the ischium. Case report. J Bone Joint Surg Am 1979;61:599-600. [Google Scholar | PubMed]
- 23.Beppu H, Yamaguchi H, Yoshimura N, Atarashi K, Tsukimoto K, Nagashima Y. Adamantinoma of the rib metastasizing to the liver. Intern Med 1994;33:441-5. [Google Scholar | PubMed]
- 24.Dini LI, Mendonca R, Adamy CA, Saraiva GA. Adamantinoma of the spine: Case report. Neurosurgery 2006;59:E426. [Google Scholar | PubMed]
- 25.Diepeveen WP, Hjort GH, Pock-Steen OC. Adamantinoma of the capitate bone. Acta radiol 1960;53:377-84. [Google Scholar | PubMed]
- 26.Bambirra EA, Nogueira AM, Miranda D. Adamantinoma of the soft tissue of the leg. Arch Pathol Lab Med 1983;107:500-1. [Google Scholar | PubMed]
- 27.Qureshi AA, Shott S, Mallin BA, Gitelis S. Current trends in the management of adamantinoma of long bones. An international study. J Bone Joint Surg Am 2000;82:1122-31. [Google Scholar | PubMed]
- 28.Altmannsberger M, Poppe H, Schauer A. An unusual case of adamantinoma of long bones. J Cancer Res Clin Oncol 1982;104:315-20. [Google Scholar | PubMed]
- 29.Van Schoor JX, Vallaeys JH, Joos GF, Roels HJ, Pauwels RA, Van Der Straeten ME. Adamantinoma of the tibia with pulmonary metastases and hypercalcemia. Chest 1991;100:279-81. [Google Scholar | PubMed]
- 30.Bridge JA, Fidler ME, Neff JR, Degenhardt J, Wang M, Walker C, et al. Adamantinoma like Ewing’s sarcoma: Genomic confirmation, phenotypic drift. Am J Surg Pathol 1999;23:159-65. [Google Scholar | PubMed]
- 31.Hauben E, van den Broek LC, van Marck E, Hogendoom PC. Adamantinoma-like Ewing’s sarcoma and Ewing’s-like adamantinoma. The t(11;22), t(21;22) status. J Pathol 2001;195:218-21. [Google Scholar | PubMed]
- 32.Folpe AL, Goldblum JR, Rubin BP, Shehata BM, Liu W, Dei Tos AP, et al. Morphologic and immunophenotypic diversity in Ewing family tumors. A study of 66 genetically confirmed cases. Am J Surg Pathol 2005;29:1025-33. [Google Scholar | PubMed]
- 33.Fujii H, Honoki K, Enomoto Y, Kasai T, Kido A, Amano I, et al. Adamantinoma-like Ewing’s sarcoma with EWS-FLI1 fusion gene: A case report. Virchows Arch 2006;449:579-84. [Google Scholar | PubMed]
- 34.Rodriguez-Galindo C, Spunt SL, Pappao AS. Treatment of Ewing Sarcoma family of tumors: Current status and outlook for the future. Med Pediatr Oncol 2003;40:276-87. [Google Scholar | PubMed]
- 35.Burdach S, Jurgens H, Peters C, Nurnberger W, Mauz-Korholz C, Korholz D, et al. Myeloablative radiochemotherapy and hematopoietic stem-cell rescue in poor-prognosis Ewing’s sarcoma. J Clin Oncol 1993;11:1482-8. [Google Scholar | PubMed]
- 36.Burdach S, Meyer-Bahlburg A, Laws HJ, Haase R, van Kaik B, Metzner B, et al. High-dose therapy for patients with primary multifocal and early relapsed Ewing’s tumors: Results of two consecutive regimens assessing the role of total-body irradiation. J Clin Oncol 2003;21:3072-8. [Google Scholar | PubMed]
- 37.Liu T, Guo X, Zhang X, Li Z, Zhang Q. Reconstruction with pasteurized autograft for primary malignant bone tumor of distal tibia. Bull Cancer 2012;99:87-91. [Google Scholar | PubMed]
- 38.Mavrogenis AF, Abati CN, Romagnoli C, Ruggieri P. Similar survival but better function for patients after limb salvage versus amputation for distal tibia osteosarcoma. Clin Orthop Relat Res 2012;470:1735-48. [Google Scholar | PubMed]
- 39.Gharedaghi M, Peivandi MT, Mazloomi M, Shoorin HR, Hasani M, Seyf P, et al. Evaluation of clinical results and complications of structural allograft reconstruction after bone tumor surgery. Arch Bone Joint Surg 2016;4:236-42. [Google Scholar | PubMed]
- 40.Kundu ZS, Gogna P, Gupta V, Singla R, Sangwan SS, Mohindra M, et al. Ankle fusion with centralisation of the fibula after distal tibia bone tumour resection. J Orthop Traumatol 2014;15:95-101. [Google Scholar | PubMed]
- 41.El-Negery A, Elmoghazy NA, Abd-Ellatif MS, Elgeidi A. Vascularized fibular medialization for reconstruction of the tibial defects following tumour excision. Int Orthop 2017;41:2179-87. [Google Scholar | PubMed]
- 42.Abudu A, Grimer RJ, Tillman RM, Carter SR. Endoprosthetic replacement of the distal tibia and ankle joint for aggressive bone tumours. Int Orthop 1999;23:291-4. [Google Scholar | PubMed]
- 43.Yang Y, Han L, He Z, Li X, Yang S, Yang J, et al. Advances in limb salvage treatment of osteosarcoma. J Bone Oncol 2018;10:36-40. [Google Scholar | PubMed]
- 44.Ozaki T, Nakatsuka Y, Kunisada T, Kawai A, Dan’ura T, Naito N, et al. High complication rate of reconstruction using Ilizarov bone transport method in patients with bone sarcomas. Arch Orthop Trauma Surg 1998;118:136-9. [Google Scholar | PubMed]
- 45.Lee SH, Kim HS, Park YB, Rhie TY, Lee HK. Prosthetic reconstruction for tumours of the distal tibia and fibula. J Bone Joint Surg Br 1999;81:803-7.[OMIT] [Google Scholar | PubMed]








