Isolated upper extremity compartment syndrome can cause severe rhabdomyolysis resulting in AKI.
Dr. Ethan Sawyer, Department of Orthopedics, University of Toledo Medical School, Toledo, Ohio, USA. E-mail: ethan.sawyer@utoledo.edu
Introduction: The association between rhabdomyolysis secondary to traumatic crush injuries and the resultant acute kidney injury has been well described [1]. The pathway of opioid overdose and acute kidney injury (AKI) has been documented but not fully elucidated [2]. This process is believed to be multifactorial in its pathophysiology, but it remains obscure [2]. Acidosis, systemic hypoxia, hypothermia, muscle compression, immunologic, or direct toxic effects have been identified as contributing factors to opioid-induced AKI [3]. Musculoskeletal crush injuries account for one of the most common causes of rhabdomyolysis leading to AKI [3]. However, the vast majority of crush injuries documented involve large regions of the body and most commonly involve the lower extremity [4]. This is hypothesized to be due to the need for a considerable amount of muscle necrosis and sufficient myoglobinuria to cause AKI [3, 4]. There is a paucity of literature describing isolated upper extremity crush injuries severe enough to cause AKI. The case described herein outlines a patient who developed isolated right upper extremity compartment syndrome and resultant rhabdomyolysis leading to AKI in the setting of an opioid overdose.
Case Report: Rhabdomyolysis may be caused by a variety of metabolic events. The pathophysiology of rhabdomyolysis secondary to acute crush injuries with resultant AKI is well documented. However, the literature describing cases of acute kidney injury caused by upper extremity compartment syndrome-induced rhabdomyolysis is limited. We present the case of a 33-year-old male who developed right upper extremity compartment syndrome after being incapacitated following an opioid overdose. He subsequently underwent emergent fasciotomies and was found to have an AKI secondary to rhabdomyolysis in the acute post-operative period.
Conclusion: This case describes a patient who was found to have isolated right upper extremity compartment syndrome and subsequent rhabdomyolysis, which resulted in AKI following an opioid overdose. This case highlights that an isolated incidence of upper extremity rhabdomyolysis is sufficient to cause acute kidney injury. The literature describing this pathology in isolated upper extremity injuries is limited as this phenomenon is typically encountered in the setting of lower extremity compartment syndrome. The pathophysiology and mechanism of this pathology are of particular importance to the fields of orthopedic surgery, nephrology, and internal medicine. This case highlights the need for early and adequate fluid resuscitation in patients with isolated upper extremity injuries to minimize the risk of subsequent AKI.
Keywords: Compartment syndrome, upper extremity, rhabdomyolysis, acute kidney injury.
The association between rhabdomyolysis secondary to traumatic crush injuries and the resultant acute kidney injury has been well described [1]. The pathway of opioid overdose and acute kidney injury (AKI) has been documented but not fully elucidated [2]. This process is believed to be multifactorial in its pathophysiology, but it remains obscure [2]. Acidosis, systemic hypoxia, hypothermia, muscle compression, immunologic, or direct toxic effects have been identified as contributing factors to opioid-induced AKI [3]. Musculoskeletal crush injuries account for one of the most common causes of rhabdomyolysis, leading to AKI [3]. However, the vast majority of crush injuries documented involve large regions of the body and most commonly involve the lower extremity [4]. This is hypothesized to be due to the need for a considerable amount of muscle necrosis and sufficient myoglobinuria to cause AKI [3,4]. There is a paucity of literature describing isolated upper extremity crush injuries severe enough to cause AKI. The case described herein outlines a patient who developed isolated right upper extremity compartment syndrome and resultant rhabdomyolysis leading to AKI in the setting of an opioid overdose.
A 33-year-old male was found alone and unresponsive on a public bathroom floor lying on top of his left arm for an indeterminant amount of time; however, it was estimated to be several hours. EMS administered Narcan to the patient, which led to minimal improvement in responsiveness. Upon evaluation in the emergency department, he was intubated and given Naloxone, hydrocortisone, and piperacillin-tazobactam. A stat CT of the head was performed, which was negative for intracranial bleed, and he was subsequently admitted to the intensive care unit for further workup. His laboratories on admission included a creatine kinase (CK) of 1547 IU/L, creatinine of 1.7 mg/d, and GFR of 56 (Tables 1 and 2). The urine toxicology screen was positive for opioids.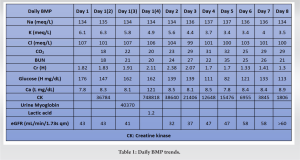
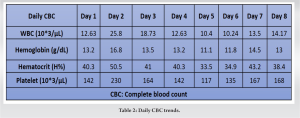
On the 2nd day of admission, the patient’s CK was noted to increase to 38640 IU/L (Table 1). The medical team also became concerned with left upper extremity swelling, and orthopedics was consulted to rule out an underlying musculoskeletal injury. The physical examination was limited by patient sedation, but he was noted to have severe swelling of the arm and forearm as well as a decreased radial pulse in comparison to the contralateral side. Compartment pressures of the left forearm were obtained using an intercompartmental measurement device. The volar compartment was found to have a pressure of 25 mmHg, the dorsal compartment was 41 mmHg, and the mobile was 25 mmHg. Based on these findings, in conjunction with a diastolic blood pressure of 80, it was decided that the patient should undergo an emergent bedside fasciotomy. Laboratories at this time demonstrated myoglobinuria, which was consistent with ischemic muscle damage. The patient underwent emergent fasciotomies within 1 h of consultation. The skin was prepped with betadine, and a volar forearm incision was made overlying the flexor carpi radialis tendon. There was moderate pallor of the superficial flexor muscles, providing further evidence of ischemic damage and muscle necrosis. Blunt dissection was carried out digitally on the underlying fascia, which was then incised. The flexor digitorum superficialis and flexor digitorum profundus were released and mobilized until the radius was encountered and all volar compartments were released. Next, the dorsal forearm compartment was released through a longitudinal incision. The dorsal musculature also showed pallor consistent with muscle necrosis. Blunt dissection was carried out to the level of the ulna, ensuring that all muscles in the dorsal compartment had been adequately released. At this point, the upper arm was noted to have moderate to severe residual swelling, although the compartments were noted to be somewhat compressible. Given the amount of tissue damage and necrosis present in the forearm, it was deemed appropriate to proceed with fasciotomies of the arm. A longitudinal anterolateral incision was made along the arm. The brachialis and coracobrachialis muscle bellies were identified and appeared to have adequate blood supply and normal contractility. All grossly necrotic tissues encountered in the forearm were debrided before the wounds were packed with sterile wet-to-dry dressings and loosely wrapped. He returned to the operating room for irrigation and debridement of his fasciotomy wounds on post-operative day 4 (Fig. 1-5).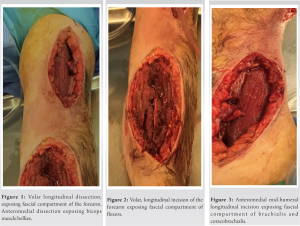
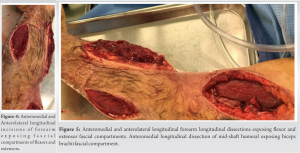 At this time, the volar forearm was noted to have further necrotic muscle, which was sharply debrided. On the 4th day of admission, his creatinine improved to 1.7 mg/dL, while his BUN was 35 mg/dL (Table 1). This further decreased on the 6th day of admission to 1.41 mg/dL and 26 mg/dL, respectively (Table 1). On post-operative day 7, he underwent a second irrigation and debridement of his wounds, which were still deemed unacceptable for primary closure. On the 8th day of admission, the patient’s creatinine and BUN had normalized (Table 1). The patient subsequently underwent split-thickness skin grafting on postoperative day 9 for wound coverage. Donor-site grafting was obtained from the patient’s left anterior thigh. At the last encounter, the patient had limited elbow and wrist range of motion. Additionally, he had minimal flexion movement and no extension in the digits of the hand, and there was residual pain and paresthesia in his left upper extremity. He was prescribed Gabapentin for neuropathic pain and referred to Physical Medicine and Rehabilitation for his paresthesia and hemiparesis. He was seen by psychiatry for his opioid addiction. He was then lost to further orthopedic follow-up.
At this time, the volar forearm was noted to have further necrotic muscle, which was sharply debrided. On the 4th day of admission, his creatinine improved to 1.7 mg/dL, while his BUN was 35 mg/dL (Table 1). This further decreased on the 6th day of admission to 1.41 mg/dL and 26 mg/dL, respectively (Table 1). On post-operative day 7, he underwent a second irrigation and debridement of his wounds, which were still deemed unacceptable for primary closure. On the 8th day of admission, the patient’s creatinine and BUN had normalized (Table 1). The patient subsequently underwent split-thickness skin grafting on postoperative day 9 for wound coverage. Donor-site grafting was obtained from the patient’s left anterior thigh. At the last encounter, the patient had limited elbow and wrist range of motion. Additionally, he had minimal flexion movement and no extension in the digits of the hand, and there was residual pain and paresthesia in his left upper extremity. He was prescribed Gabapentin for neuropathic pain and referred to Physical Medicine and Rehabilitation for his paresthesia and hemiparesis. He was seen by psychiatry for his opioid addiction. He was then lost to further orthopedic follow-up.
There is a paucity of literature reporting on isolated upper extremity injuries leading to compartment syndrome, rhabdomyolysis, and subsequent AKI. Acute limb compartment syndrome occurs when there is an increase in pressure in a closed fascial compartment [5]. Compartment syndrome is usually caused by an event that disrupts the venous outflow and arterial inflow such as tissue injury, burns, vascular injury, crush injuries, improperly placed casts and splints, tight bandaging, intense athletic activity, or drug overdoses, which was the case in this patient. This process can lead to ischemia of muscles and nerves due to bleeding and/or edema in a closed muscle compartment and is a surgical emergency [5]. Cellular ischemia in the context of muscle fibers can lead to rhabdomyolysis, tissue ischemia, and necrosis [1]. Opiate overdose has been associated with rhabdomyolysis through an unclear, multi-factorial mechanism including acidosis, systemic hypoxia, hypothermia, muscle compression, immunologic, or direct toxic effects [6,7,8]. Opioids acting through several mechanisms, including Mu, Kappa, and Delta agonists, can affect kidney function. Kappa agonists in experimental data have demonstrated potential therapeutic efficacy in water retention and hyponatremia [9]. In the instance of this patient’s injury, pressure from lying on his right upper extremity for an unknown period impeded blood flow, leading to compartment syndrome and subsequent rhabdomyolysis. The isolated nature of his compartment syndrome and timing correlate with his degree of rhabdomyolysis. The disease process of rhabdomyolysis is well documented: degradation of striated skeletal muscle fibers causes the release of proteins and electrolytes into circulation and extracellular fluid, leading to its characteristic clinical and laboratory manifestations [1,3]. Rhabdomyolysis can be caused by numerous processes, including trauma-related crush injury, exertion, muscle hypoxia, genetic defects, infections, changes in body temperature, metabolic and electrolyte dysregulation, drugs, and toxins, as well as other additional causes [9]. This process may initially present with varying levels of severity, from isolated elevated CK with muscle weakness and myalgias to life-threatening AKI and disseminated intravascular coagulation [1,3]. Some of the proteins released into the circulation during rhabdomyolysis include myoglobin, creatine phosphokinase, lactate dehydrogenase, alanine aminotransferase, aspartate aminotransferase, and aldolase [1,9]. A CK level >5000 U/I in the absence of concomitant myocardial or brain infarcts is the most sensitive laboratory marker for rhabdomyolysis [9]. In the case of this patient, the CK reached 748.818 HH IU/L. Of note, myoglobinuria typically occurs rarely outside of rhabdomyolysis, as myoglobin is otherwise not present in the urine [9]. Once the renal threshold of 0.5–1.5 mg of myoglobin/dL is exceeded, myoglobin is able to enter the urine, which classically leads to six grossly tea-colored urines at 100 mg/dL. In this patient, urine myoglobin was measured at 40.370 HH ng/mL. Intracellular electrolytes may also be released during rhabdomyolysis, most notably potassium, which can cause a variety of systemic effects, including arrhythmia [1,6,9]. In this patient, at presentation, his potassium was 6.1 mEq/L, which, after adequate hydration, decreased to 5.8 mEq/L. Hypocalcemia may also occur following rhabdomyolysis, although it is often not corrected immediately to avoid potential calciphylaxis. Rhabdomyolysis-induced AKI, pigment nephropathy, or crush kidney injury develops following skeletal muscle trauma associated with physical, thermal, ischemic, infective, endocrinologic, toxic, or metabolic causes and is associated with high morbidity and mortality [1,3,6,9]. Muscular and physical trauma are the most common causes of rhabdomyolysis, as was the case in our patient. The mechanism of glomerular filtration impairment due to rhabdomyolysis is obscure but likely due to vasoconstriction, direct ischemic tubular injury, and tubular obstruction [9]. If myoglobinuria is sufficiently high, AKI commonly results [3]. AKI is a heterogenous set of conditions characterized by a sudden decrease in glomerular filtration rate, which may be identified by an increase in serum creatinine concentration or oliguria [3,8,10]. Rhabdomyolysis is one of the most common causes of AKI, constituting 7–10% of the injuries [9]. AKI is further classified by stage and cause of injury. The pathophysiology of AKI secondary to rhabdomyolysis has not been fully elucidated but likely involves several pathways that are most associated with myoglobinuria [8,9]. AKIs resulting from crush injuries have been documented as early as 1908 in German military literature and throughout the Second World War [4,9,10]. However, there is insufficient literature to date describing isolated upper extremity incidents leading to the development of AKI. In the event of rhabdomyolysis, intrarenal vasoconstriction, direct and ischemic tubular injury, as well as tubular obstruction, all play a role in AKI [3]. Myoglobin congregates along the renal tubules, which is enhanced during volume depletion [3,10]. Obstruction occurs primarily at the level of the distal tubules, while direct cytotoxicity occurs in the proximal tubules [3]. This complex can form tubular casts, which may act as a nitric oxide scavenger and generate reactive oxygen species (ROS) [3]. ROS cause tubular toxicity and vasoconstriction, contributing to decreased renal cortical blood flow [2]. Muscle swelling, intravascular volume depletion, lactic acidosis, and decreased cardiac output can all contribute to AKI [11]. AKI is common, occurring in approximately 20% of hospitalized patients [9,12,13]. Since this disease state can be caused by a variety of conditions, treatment is specific to the underlying condition and is largely supportive and symptomatic in nature [3,8,9,12,13]. If medical management is not feasible and chronic renal failure ensues, dialysis can be used as a treatment option until ultimate kidney transplantation. Literature discussing isolated upper-limb ischemia leading to AKI is lacking. In our patient, isolated upper-limb ischemia was severe enough to lead to myoglobinuria and cause AKI. Surgeons caring for trauma patients with ischemic injuries should remain vigilant for these potential complications and ensure the early involvement of the medical and nephrology teams.
This case describes a patient who was found to have isolated right upper extremity compartment syndrome and subsequent rhabdomyolysis, which resulted in AKI following an opioid overdose. This case highlights that an isolated incidence of upper extremity rhabdomyolysis is sufficient to cause acute kidney injury. The literature describing this pathology in isolated upper extremity injuries is limited as this phenomenon is typically encountered in the setting of lower extremity compartment syndrome. The pathophysiology and mechanism of this pathology are of particular importance to the fields of orthopedic surgery, nephrology, and internal medicine. This case highlights the need for early and adequate fluid resuscitation in patients with isolated upper extremity injuries to minimize the risk of subsequent AKI.
Fluid resuscitation is important in upper extremity compartment syndrome due to the possibility of rhabdomyolysis-induced AKI.
References
- 1.Vanholder R, Sever MS, Erek E, Lameire N. Rhabdomyolysis. J Am Soc Nephrol 2000;11:1553-61. [Google Scholar | PubMed]
- 2.Mercadante S, Arcuri, E. Opioids and renal function. J Pain 2004;5:2-19. [Google Scholar | PubMed]
- 3.Rosenberger C, Goldfarb M, Shina A, Bachmann S, Frei U, Eckardt KU, et al. Evidence for sustained renal hypoxia and transient hypoxia adaptation in experimental rhabdomyolysis-induced acute kidney injury. Nephrol Dialysis Transplant 2007;23:1135-43. [Google Scholar | PubMed]
- 4.Bywaters EG, Beall D. Crush injuries with impairment of renal function. Br Med J 1941;1:427-32. [Google Scholar | PubMed]
- 5.Torlincasi AM, Lopez RA, Waseem M. Acute Compartment syndrome. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2019. [Google Scholar | PubMed]
- 6.Brudvig T, Fitzgerald P. Identification of signs and symptoms of acute exertional rhabdomyolysis in athletes: A guide for the practitioner. Strength Cond J 2007;29:10-4. [Google Scholar | PubMed]
- 7.Melandri R, Re G, Lanzarini C, Rapezzi C, Leone O, Zele I, Rocchi G. Myocardial damage and rhabdomyolysis associated with prolonged hypoxic coma following opiate overdose. J Toxicol Clin Toxicol 1996;34:199-203. [Google Scholar | PubMed]
- 8.Kumar R, West DM, Jingree M, Laurence AS. Unusual consequences of heroin overdose: Rhabdomyolysis, acute renal failure, paraplegia and hypercalcaemia. Br J Anaesth 1999;83:496-8. [Google Scholar | PubMed]
- 9.Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. New Engl J Med 2009;361:62-72. [Google Scholar | PubMed]
- 10.Huerta-Alardín AL, Varon J, Marik PE. Bench-to-bedside review: Rhabdomyolysis--an overview for clinicians. Crit Care 2004;9:158-69. [Google Scholar | PubMed]
- 11.Hsu CH, Kurtz TW, Waldinger TP. Cardiac output and renal blood flow in glycerol-induced acute renal failure in the rat. Circ Res 1977;40:178-82. [Google Scholar | PubMed]
- 12.Brown JA, Elliott MJ, Sray WA. Exercise-induced upper extremity rhabdomyolysis and myoglobinuria in shipboard military personnel. Mil Med 1994;159:473-5. [Google Scholar | PubMed]
- 13.Moratalla MB, Braun P, Fornas GM. Importance of MRI in the diagnosis and treatment of rhabdomyolysis. Eur J Radiol 2008;65:311-5. [Google Scholar | PubMed]










