Severe multifocal systemic and spinal epidural abscesses and septic arthritis require extensive pre-operative optimization, management by a multidisciplinary team, and selective surgical drainage to avoid excessive surgical stress.
Dr. Cassie Yang, Department of Orthopaedic Surgery, Singapore General Hospital, 1 Outram Road, Singapore 169608. E-mail: cassie.yang@mohh.com.sg
Introduction: Multiple spinal epidual abscesses with multifocal systemic abscess and multiple joint septic arthritis present with a large infective burden resulting in sepsis, systemic inflammatory dysregulation, and multi-organ failure. This requires pre-operative resuscitation and surgery of greater complexity, longer operative duration, and blood loss, creating challenges to surgical management.
Case Report: A 69-year-old Chinese female presented with multilevel discrete spinal epidural abscesses along the cervical, thoracic, and lumbar spine, alongside concomitant multifocal systemic abscesses and multiple small joint septic arthritis. She received pre-operative resuscitation to restore organ function, reverse acidosis and coagulopathy, before surgical decompression of selected abscesses and joints under a multidisciplinary team. Remaining sites of infection without significant compression were undrained. The patient recovered well with no residual neurological deficits.
Conclusion: Multifocal infections in critically ill patients require a multidisciplinary team for preoperative resuscitation, joint surgical planning, and prioritizing surgical interventions to prevent excessive surgical stress to the patient.
Keywords: Spinal epidural abscess, multiple soft-tissue abscesses, septic arthritis.
Unifocal soft-tissue abscesses are typically treated with uncomplicated surgical decompression. Multifocal abscesses are rare, especially in the spine, and complicate the management by presenting a larger infective burden resulting in systemic inflammatory dysregulation. Their locations necessitate surgery of greater complexity, longer operative duration, and blood loss. The authors report a case of discrete multi-level cervicothoracolumbar spinal epidural abscesses (SEAs) with concomitant multifocal systemic abscesses and multiple joint septic arthritis, complicated by septicemia and multiple organ compromise. The patient had a background of untreated cervical myelopathy with acutely worsening weakness. This patient received initial resuscitation and perioperative antibiotics, only undergoing selective surgical decompression after adequate optimization under a multi-disciplinary team. This triaged approach was adopted due to higher mortality being observed after multiple surgical interventions were applied indiscriminately on critically ill patients with systemic inflammatory dysfunction, also commonly seen in polytrauma [1]. This treatment model advocates for initial resuscitation and a triage of interventions to avoid the effects of excessive surgical stress, akin to a “surgical 2nd hit” [2].
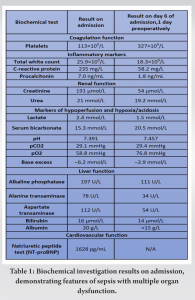
A 69-year-old female with Type 2 diabetes mellitus presented to the emergency department following a fall, secondary to bilateral lower limb weakness, with urinary retention and constipation for 3 days. There was proximal weakness over the upper (power 4/5) and lower limbs (power 3/5), intact anal tone, and no saddle anesthesia. She was afebrile but hypoxic with raised jugular venous pressure, lung bibasal crepitations, and bilateral lower limb oedema. Initial investigations revealed high C-reactive protein (CRP), high anion gap metabolic acidosis, acute renal failure, hepatic dysfunction, and thrombocytopenia. She was admitted to internal medicine with a diagnosis of sepsis and received initial resuscitation with empiric intravenous (IV) ceftriaxone and metronidazole, intranasal oxygen (O2), IV furosemide, and judicious crystalloids, without requiring inotropic support. Methicillin-Sensitive Staphylococcus Aureus (MSSA) was detected in blood cultures, prompting a change of antibiotics to IV cloxacillin. Subsequently, her hepatic and renal dysfunction improved, with normalization of lactate, base excess, and platelet count within 1 week (Table 1).
.
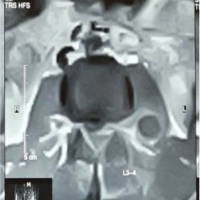
Her inflammatory markers remained elevated with worsening proximal weakness (power 3/5 over upper limbs and 2 to 3/5 over lower limbs) and a new onset of unrelenting back pain with cervicothoracic tenderness. A magnetic resonance imaging (MRI) study of the full spine found SEAs at levels C3/4, C6-7, T5 to T7, L1 to S2 levels, with central canal narrowing and severe cord compression by a large degenerative osteochondral bar at C5/6 with focal cystic gliosis. There was associated severe thecal sac and cauda equina compression from L2-3 to L4-5 levels. The T5 to T7 collections displayed mild indentation with no significant compression of the spinal cord. There was C3/4 and C6/7 infective spondylodiscitis and osteomyelitis, septic arthritis of left C3/4 and C4/5 facet joints, T5 and T6 costovertebral joint septic arthritis and osteomyelitis, bilateral loculated iliopsoas abscesses at L5 level and a right thigh abscess.
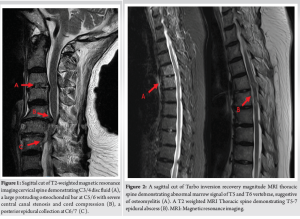
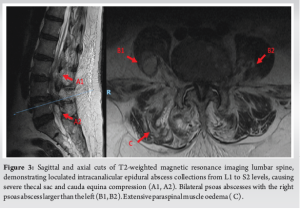
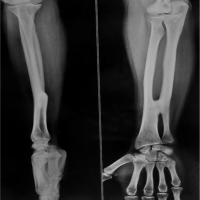
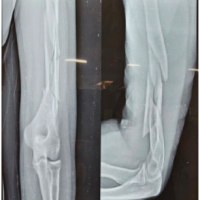
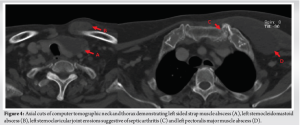
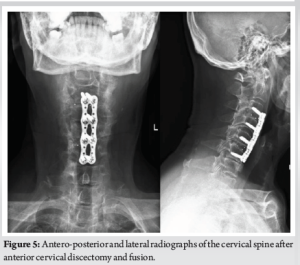
(Fig. 1, 2, 3) A computer tomographic (CT) scan of the thorax, abdomen and pelvis demonstrated a left pectoralis major abscess and left strap muscle abscesses, left sternoclavicular, and first sternocostal joint septic arthritis. A transthoracic cardiac echogram did not demonstrate any vegetations suggestive of infective endocarditis while a detailed head-to-toe examination did not reveal any obvious skin or dental lesions. The urinary analysis and urinary culture were unremarkable although the patient was also found to have chronic left obstructive hydronephrosis. The patient had no history of IV drug abuse. A multidisciplinary team comprising of specialists from internal medicine, cardiology, infectious diseases, anesthesia, otolaryngology, cardiothoracic surgery, urology, orthopedic, and spine surgery was assembled for pre-operative medical optimization. They also developed joint goals of surgery as well as a detailed surgical plan on the day of surgery and post-operative care. The patient underwent concurrent drainage of the left pectoralis major and left strap muscle abscesses, followed by C3/4, C5/6 anterior cervical discectomy and fusion (Fig. 4) in a supine position. The patient was subsequently turned prone for the drainage of lumbar SEAs with right L2/3, left L3/4, laminotomy, L5 and S1 laminectomy. The alternate-sided laminotomies were performed to preserve the contralateral structures to maintain spinal stability. Finally, the right psoas and right thigh abscesses were drained in a left lateral position. The thoracic SEAs and left psoas abscess were left undrained. The septic arthritis of left C3/4, C4/5 facet joints, T5/6, T6/7 costovertebral and left sternoclavicular, and first sternocostal joint was also conservatively managed.
Surgical drains were inserted into the cervical, lumbar, right psoas, left strap muscles, and left chest wall regions. These procedures were carried out by the spine, cardiothoracic, and otolaryngology service, with multiple surgeons operating simultaneously. The total operating time was 7 h and 40 min, with total time under general anesthesia of 11 h and 40 min. Intraoperative coagulopathy and excessive bleeding were addressed with 1g of IV Tranexamic acid, 4 units of packed cell transfusion (PCT), and 2 units of fresh frozen plasma (FFP) and multiple vasopressors. Postoperatively, the patient was admitted to the high dependency ward and reviewed 24 h later for a “second-look” surgical recommendation, which was not required.
The patient had markedly improved numbness on post-operative day (POD) 1 and was transferred to the general ward on POD 2. Her drain outputs decreased steadily, with her cervical, lumbar and psoas drains removed on POD 6. Her neck and chest wall drains were removed on POD 12 and POD 20. A repeat contrasted CT Neck, thorax, abdomen, and pelvis was performed at POD 43 demonstrated interval improvement of all collections. The MRI of her spine was not repeated in view of her clinical improvement and return of neurological function. She completed 7 weeks of IV antibiotics as guided by the downward trend in her serial biochemical markers of sepsis and was discharged with long-term suppression oral clindamycin. One year after surgery, the patient was deemed to have recovered as evidenced by her functional status as independently ambulant, pain free, and with no neurological symptoms. Repeat biochemical markers of sepsis demonstrated a consistently normal CRP (0.4 mg/L), ESR (7 mm/Hr) and total white count (TWC) (6.3 × 109/L) (Fig 5).
This patient presented upper and lower limb weakness, lethargy, and elevated biochemical markers for sepsis including evidence of organ failure. The presumptive diagnosis of sepsis was made with the suspicion of a spinal infection. Spinal infections are responsible for 2–7% of all musculoskeletal infections with an incidence of one in 100,000–250,000 in developed countries [3], typically as spondylodiscitis, vertebral osteomyelitis, or epidural abscesses. Multilevel SEAs are an exceedingly rare subset of this entity, with only 1% demonstrating synchronous SEAs involving the cervical, thoracic, and lumbar regions in a meta-analysis of 915 cases [4]. The typical triad for SEAs is back pain, fever, and neurological deficit. The presence of neurological deficits is often a late and irreversible sign [5], while fever may be absent, rendering this an unreliable diagnostic tool. Skip lesions may be missed as symptoms might mistakenly be attributed to a single affected area of the spine [6], especially if there is both upper and lower limb neurological deficits. An early gadolinium-contrasted MRI of the whole spine was performed once spinal infection was suspected to avoid missing out skip lesions while a contrasted CT of the chest, abdomen, and pelvis sought for other soft tissue and deep collections. These investigations demonstrated multi-level SEAs and multifocal systemic abscesses and multi-joint septic arthritis. Given the disseminated sites of infection, the nature of seeding appeared to be hematogenous, the most common mode of spread [7]. The patient had bacteremia with positive blood cultures of MSSA, the most common organism in SEAs. The same organism was found in cultures of the pus collected from the various sites of infection. Other common organisms in SEAs include coagulase-negative Staphylococcus, Streptococcus species, and Gram-negative bacteria [7]. Due to the large infective burden, the patient was in sepsis complicated by septic shock. Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection [8]. Septic shock is a subset of sepsis which causes a profound global hypoperfusion from macro and microcirculatory dysfunction resulting in multi-organ failure [9]. The prognosis of SEAs is poor due to its risk of high morbidity and mortality [10]. The overall 30-day mortality rate of SEA is 3.7%. In patients with 4 or more independent risk factors of mortality (age >60, diabetes, renal comorbidity, pre-operative thrombocytopenia, and septic shock), the mortality rate can reach 37.5% [11]. Although there have been reports of extensive SEAs resolving with antibiotic therapy alone [12], any neurological deficit without complete para- or quadriplegia should warrant surgical intervention if the patient is a suitable surgical candidate [13]. Surgical intervention should aim to preserve neurological function and decrease the overall infective burden to allow antibiotics and the immune system to overcome the infection. Contiguous multi-level SEAs have demonstrated successful eradication with apical laminectomies through catheter irrigation through fogarty catheters or skip laminotomies with epidural catheter irrigation via infant feeding tubes [14-16]. In contrast, discrete multi-level SEAs require separate areas of drainage, conferring a high degree of surgical invasiveness, long operative duration and blood loss. A multidisciplinary team modeled after a care bundle developed to manage necrotising soft-tissue infections [17] was assembled to resuscitate the patient before surgery as well as to plan for surgery. With many therapeutic considerations with time sensitivities, the orthopedic and spinal team emerged as the overall team leader in the centralized care planning [18] in ensuring the timely optimization of the patient while leading discussions among the multiple stakeholders such as the cardiothoracic, otolaryngology, and anesthetic teams for surgical planning. The multidisciplinary team opted to prioritize (1) the drainage of significant collections to reduce infective burden, (2) protecting neurological function, and (3) avoiding extensive surgery to reduce surgical stress [19] and to minimize operating time while still achieving reasonable outcomes. The “game plan” on the day of the surgery was meticulously organized to ensure smooth flow within the theatre with multiple surgical teams operating concurrently to reduce operative time. The teams took account of the sites to be drained and their priorities before deciding on the final order of drainage. Accessing these drainage sites required the patient to be repositioned thrice leading to natural points of termination in the event of hemodynamic instability. Anesthesia was notified early of the risk of blood loss and IV tranexamic acid was administered prophylactically to mitigate against coagulopathy. PCT and FFP were reserved and ultimately administered. Despite extensive pre-operative planning, the overall surgical procedure required a long time under anesthesia of 11 h and 40 min. This was due to multiple position changes and repeated episodes of surgical cleaning and draping. Postoperatively, a repeat contrasted CTs of affected areas should be performed at 6 weeks after surgery to assess for residual undrained collections which trigger a reoccurrence [20]. For the spinal column, the decision to repeat MRI imaging should be guided by the clinical progress of the patient, taking into consideration vital signs such as fever or hypotension, biochemical markers of sepsis (CRP, ESR, and TWC), and neurological function. In the event of any neurological deterioration, a repeat pan spinal MRI should be performed to assess for the recurrence of the primary SEAs or the presence of skip lesions. For the post-surgical treatment of SEAs, the duration of antibiotics typically ranges from 4 to 8 weeks although the optimal duration, or mode of delivery, of antibiotics has yet to be established [21]. The presence of associated psoas abscesses [22] and bacteremia [23] appears to be risk factors for prolonged antibiotic use (>8 weeks). In consultation with the infectious diseases team, this patient was placed on long term antibiotic suppression beyond her initial 7 weeks of IV antibiotics. This was tailored to account for her past medical history of diabetes which is known risk factor of poor outcomes [11] and initial presentation with multifocal systemic abscesses, psoas abscesses, bacteremia, and the presence of metallic implants. The final decision to terminate long-term antibiotic suppression should be individualized to the patient.
Multifocal spinal epidural and systemic abscesses drive a systemic inflammatory response leading to multiorgan failure and require extensive pre-operative optimization. A multidisciplinary team is recommended to develop a joint surgical plan to reduce total operative time and overall surgical stress to the patient. Surgical targets within the spine should be prioritized by size and extent of neurological compression to reduce infectious burden while maximizing neurological recovery.
SEAs with simultaneous soft tissue abscesses and septic arthritis have high morbidity and mortality. Prompt multidisciplinary surgical optimization and surgical planning is crucial to remove infective burden to reduce the septic load, while also ensuring the patient does not succumb to excessive surgical stress. With good preoperative optimization and well-planned early surgical decompression, the patient can have an excellent outcome and maximal neurological recovery. Repeat imaging with contrasted CT scans of the affected areas are recommended at 6-week post-surgery to assess for residual undrained collections. MRI imaging of the spine may be deferred in patients who are clinically improving.
References
- 1.Nicola R. Early total care versus damage control: Current concepts in the orthopedic care of polytrauma patients. ISRN Orthop 2013;2013:329452. [Google Scholar | PubMed]
- 2.Santolini E, Stella M, Divano S, Ceccarelli M, Vicenti G, Bizzoca D, et al. Optimum timing of conversion from DCO to definitive fixation in closed fractures of the lower limb: When and how? Injury 2023;54:S63-9. [Google Scholar | PubMed]
- 3.Duarte RM, Vaccaro AR. Spinal infection: State of the art and management algorithm. Eur Spine J 2013;22:2787-99. [Google Scholar | PubMed]
- 4.Reihsaus E, Waldbaur H, Seeling W. Spinal epidural abscess: A meta-analysis of 915 patients. Neurosurg Rev 2000;23:175-204. [Google Scholar | PubMed]
- 5.Davis DP, Wold RM, Patel RJ, Tran AJ, Tokhi RN, Chan TC, et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med 2004;26:285-91. [Google Scholar | PubMed]
- 6.Ju KL, Kim SD, Melikian R, Bono CM, Harris MB. Predicting patients with concurrent noncontiguous spinal epidural abscess lesions. Spine J 2015;15:95-101. [Google Scholar | PubMed]
- 7.Arko L 4th, Quach E, Nguyen V, Chang D, Sukul V, Kim BS. Medical and surgical management of spinal epidural abscess: A systematic review. Neurosurg Focus 2014;37:E4. [Google Scholar | PubMed]
- 8.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016;315:801-10. [Google Scholar | PubMed]
- 9.Gorecki G, Cochior D, Moldovan C, Rusu E. Molecular mechanisms in septic shock (Review). Exp Ther Med 2021;22:1161. [Google Scholar | PubMed]
- 10.Khanna RK, Malik GM, Rock JP, Rosenblum ML. Spinal epidural abscess: Evaluation of factors influencing outcome. Neurosurgery 1996;39:958-64. [Google Scholar | PubMed]
- 11.Du JY, Schell AJ, Kim CY, Trivedi NN, Ahn UM, Ahn NU. 30-day mortality following surgery for spinal epidural abscess: Incidence, risk factors, predictive algorithm, and associated complications. Spine (Phila Pa 1976) 2019;44:E500-9. [Google Scholar | PubMed]
- 12.Duc C, Grange L, Gaudin P, Brun F, Terki RK, Barbier LP, et al. Extensive primary epidural abscess. Report of a case. Joint Bone Spine 2002;69:312-5. [Google Scholar | PubMed]
- 13.Grieve JP, Ashwood N, O’Neill KS, Moore AJ. A retrospective study of surgical and conservative treatment for spinal extradural abscess. Eur Spine J 2000;9:67-71. [Google Scholar | PubMed]
- 14.Lau D, Maa J, Mummaneni PV, Chou D. Holospinal epidural abscess. J Clin Neurosci 2014;21:517-20. [Google Scholar | PubMed]
- 15.Abd-El-Barr MM, Bi WL, Bahluyen B, Rodriguez ST, Groff MW, Chi JH. Extensive spinal epidural abscess treated with “apical laminectomies” and irrigation of the epidural space: Report of 2 cases. J Neurosurg Spine 2015;22:318-23. [Google Scholar | PubMed]
- 16.Ahuja K, Das L, Jain A, Meena PK, Arora SS, Kandwal P. Spinal holocord epidural abscess evacuated with double thoracic interval laminectomy: A rare case report with literature review. Spinal Cord Ser Cases 2019;5:62. [Google Scholar | PubMed]
- 17.Urbina T, Hua C, Sbidian E, Bosc R, Tomberli F, Lepeule R, et al. Impact of a multidisciplinary care bundle for necrotizing skin and soft tissue infections: A retrospective cohort study. Ann Intensive Care 2019;9:123. [Google Scholar | PubMed]
- 18.Bach JA, Leskovan JJ, Scharschmidt T, Boulger C, Papadimos TJ, Russell S, et al. The right team at the right time - Multidisciplinary approach to multi-trauma patient with orthopedic injuries. Int J Crit Illn Inj Sci 2017;7:32-7. [Google Scholar | PubMed]
- 19.Guerado E, Bertrand ML, Cano JR, Cerván AM, Galán A. Damage control orthopaedics: State of the art. World J Orthop 2019;10:1-13. [Google Scholar | PubMed]
- 20.Park KH, Cho OH, Lee JH, Park JS, Ryu KN, Park SY, et al. Optimal duration of antibiotic therapy in patients with hematogenous vertebral osteomyelitis at low risk and high risk of recurrence. Clin Infect Dis 2016;62:1262-9. [Google Scholar | PubMed]
- 21.Vakili M, Crum-Cianflone NF. Spinal epidural abscess: A series of 101 cases. Am J Med 2017;130:1458-63. [Google Scholar | PubMed]
- 22.Chae HJ, Kim J, Kim C. Clinical characteristics of spinal epidural abscess accompanied by bacteremia. J Korean Neurosurg Soc 2021;64:88-99. [Google Scholar | PubMed]
- 23.Patel AR, Alton TB, Bransford RJ, Lee MJ, Bellabarba CB, Chapman JR. Spinal epidural abscesses: Risk factors, medical versus surgical management, a retrospective review of 128 cases. Spine J 2014;14:326-30. [Google Scholar | PubMed]