[box type=”bio”] What to Learn from this Article?[/box]
leiomyoma could be a possible differential diagnosis of any intraosseous lesion with a gradually worsening and long-standing pain despite of benign imaging characteristics and orthopaedic oncologists need to keep this in mind in order not to miss it. Though different histological patterns of leiomyoma exist, there is no difference in the prognosis or treatment options which include wide excision with autologous or artificial bone grafting.
Case Report | Volume 6 | Issue 2 | JOCR April-June 2016 | Page 81-85| Ahmed Hamed Kassem Abdelaal, Norio Yamamoto, Katsuhiro Hayashi, Akihiko Takeuchi, Hiroyuki Tsuchiya. DOI: 10.13107/jocr.2250-0685.448
Authors: Ahmed Hamed Kassem Abdelaal[¹]'[²], Norio Yamamoto[¹], Katsuhiro Hayashi[¹], Akihiko Takeuchi[¹], Hiroyuki Tsuchiya[¹]
[1]Department of Orthopedic Surgery, Graduate School of Medical Science, Kanazawa University, Japan.
[2]Department of Orthopedic Surgery, faculty of medicine, Sohag University, Egypt.
Address of Correspondence
Dr. Ahmed Hamed Kassem Abdelaal,
Department of Orthopedic Surgery, Graduate School of Medical Science, Kanazawa University, Japan.
E-mail: ahmedallam200@hotmail.com
Abstract
Introduction: Leiomyoma is benign smooth-muscle tumor most commonly arising in the uterus, the gastrointestinal tract, and the skin. Leiomyomata are infrequently seen in the extremities and rarely seen in the bone. It is usually presented by a gradually increasing pain with non-specific radiological findings, and could be a differential diagnosis for wide range of bone tumors.
Case presentation: We report a case of a 73-year-old Japanese female patient with a painful intraosseous leiomyoma involving the proximal tibia. The patient had undergone tumor excision with wide margin, immediate weight bearing was allowed, pain had been relieved and the patient was satisfied with no recurrence, malignant change, distant metastases or functional impairment. We reviewed all published cases of intraosseous leiomyomata in English literature.
Conclusion: Diagnosis of Intraosseous leiomyoma of the extremities is difficult due to extreme rarity of the tumor and absence of pathognomonic radiological sign in X-ray, CAT, or even MRI. While the exact diagnosis is only achieved by histopathological examination and with immunohistochemistry stains, which can differentiate it from malignancy, especially from the much less rare leiomyosarcoma.
Orthopedic oncologists have to include this rare benign tumor in the differential diagnosis of any intraosseous lesion with gradually worsening and long-standing pain, despite of benign imaging characters. Different histological patterns of leiomyoma do exist, however there is no difference in prognosis or treatment options. Treatment standard includes wide excision with autologous bone graft whenever possible. Internal fixation may be necessary if the bone defect is large or there is thinning out of the cortex that may lead to pathological fracture.
Keywords: Intraosseus, Benign bone tumors, leiomyoma.
Introduction
Leiomyoma is a benign smooth-muscle tumor that most commonly arises in the uterus, the gastrointestinaltract, and the skin. It constitutes 70% to 80% of all benign mesenchymal tumors [1], with uterine leiomyomata being the most common smooth-muscle tumor in women [1]. Leiomyomata are infrequently seen in the extremities and rarely seen in bone [2]. To the best of our knowledge, the eleven reported cases occurred in peripheral skeleton. Five cases were intraosseous lesion in long bones, including neck of the femur [3],ulna [4], fibula [5], tibia [6] and distal femur [7]. Three cases were periosteal leiomyomata[8] and two cases arose from the hip bone [6]. In addition to three cases of intraosseous leiomyomatosis secondary to disseminated leiomyomatosis have been reported [9]. We describe a case of a patient with painful intraosseous leiomyoma involving the proximal aspect of the tibia and review the literature about intraosseous leiomyoma.
Case Presentation
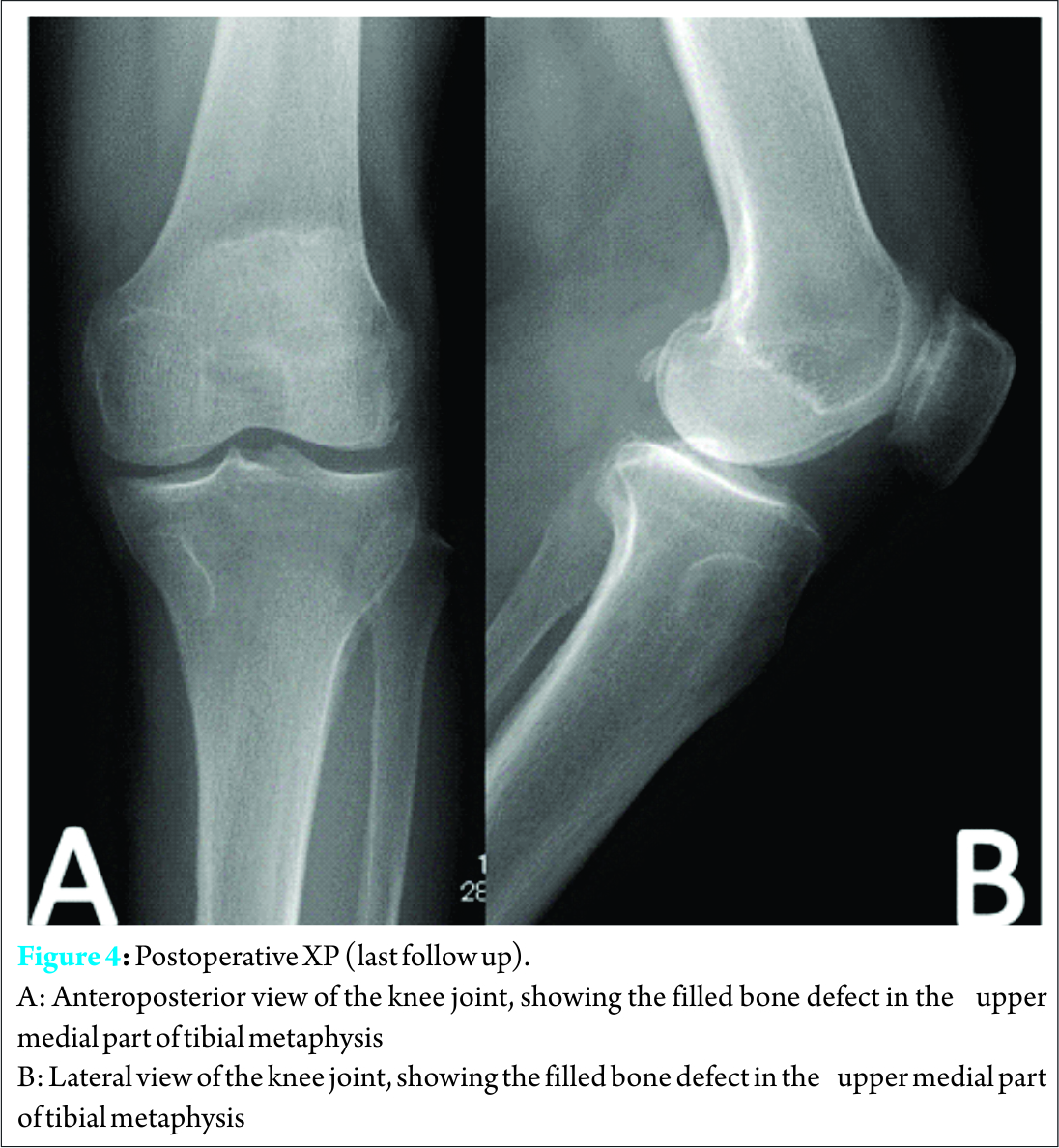
A 73-year-old female presented with a four year history of a painful swelling in the left knee that was slowly growing, with a gradual increase in pain at the same time.. She had unnoticeable medical history. Pain was aggravated by weight bearing, sitting straight, but she enjoyed full range of motion, and her general health was not affected. Osteoarthritis of left knee joint was suspected in another clinic, and she was treated conservatively with analgesic drugs. The pain persisted despite administration of nonsteroidal anti-inflammatory drugs (NSAIDs) for long time; she visited our outpatient clinic for consultation. Clinical examination revealed local tenderness over medial aspect of proximal tibia, small well defined swelling over the medial proximal tibia was palpable. It was firm in consistency and was not red or hot to touch. Roentgenography revealed a small well-defined osteolytic lesion with thinning out of the cortex over medial part of proximal tibia, surrounded by a thin sclerotic margin (Fig.1a,1b). Computed tomography (CT) scan revealed radiolucent lesion eroding the cortex, with central calcification (Fig.1c,1d)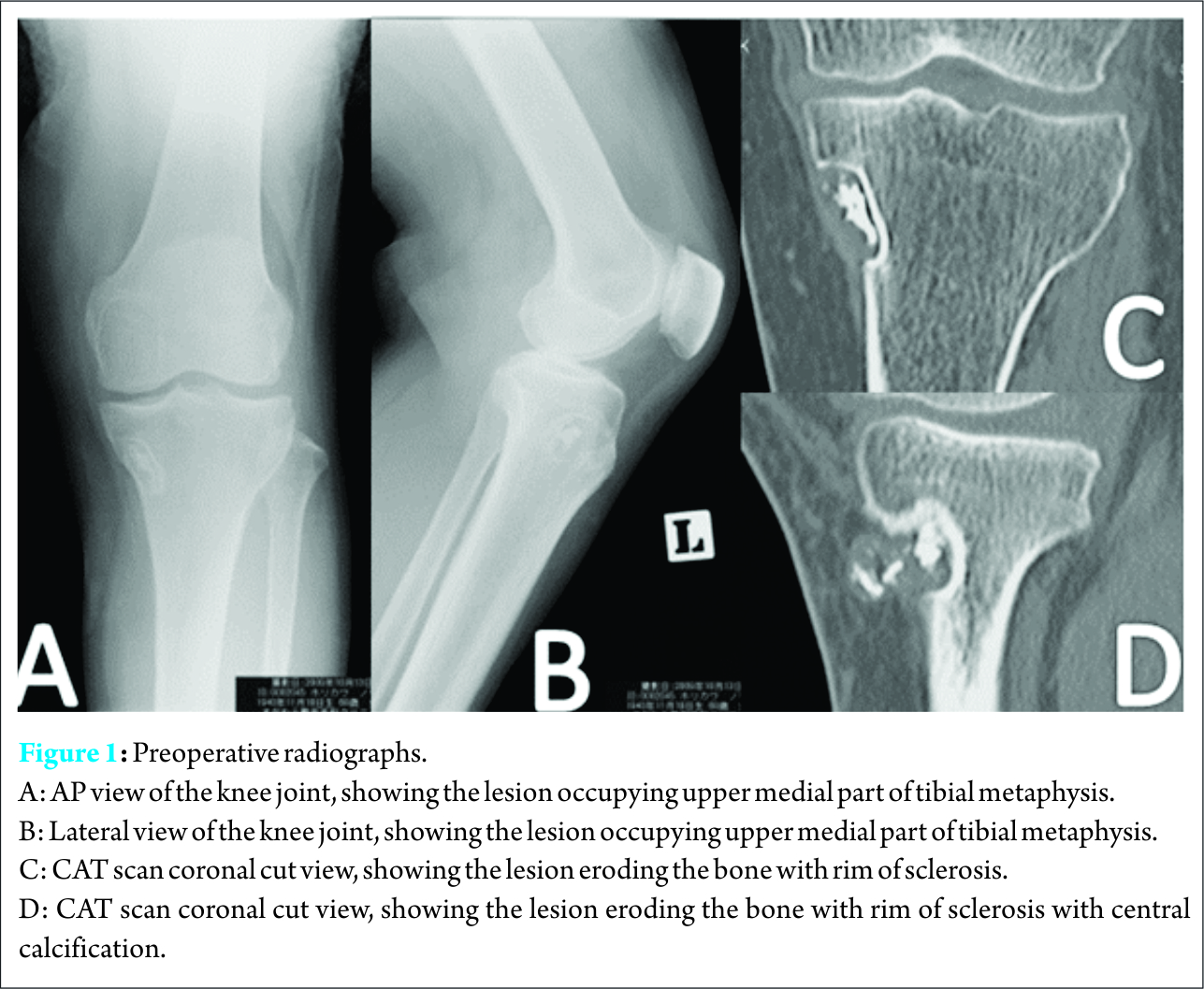
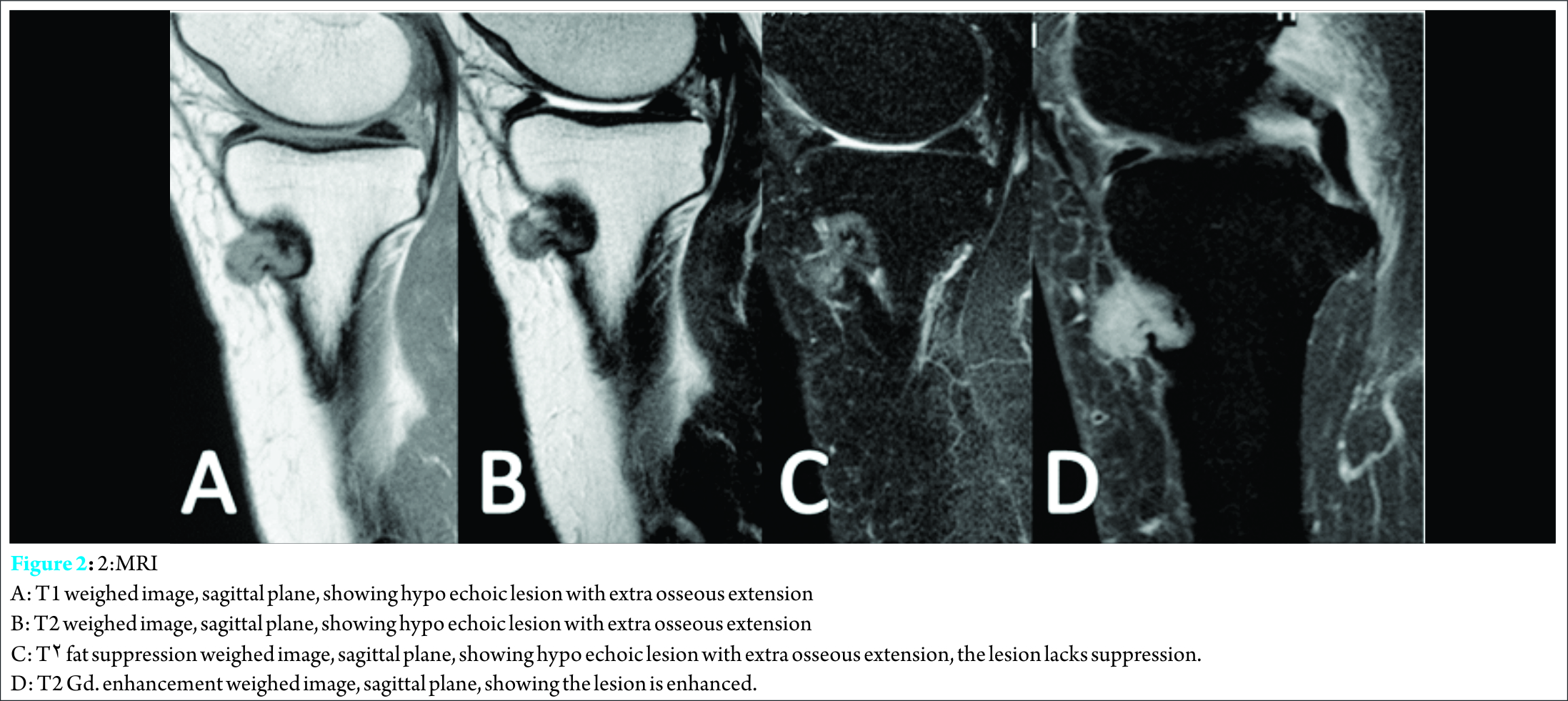
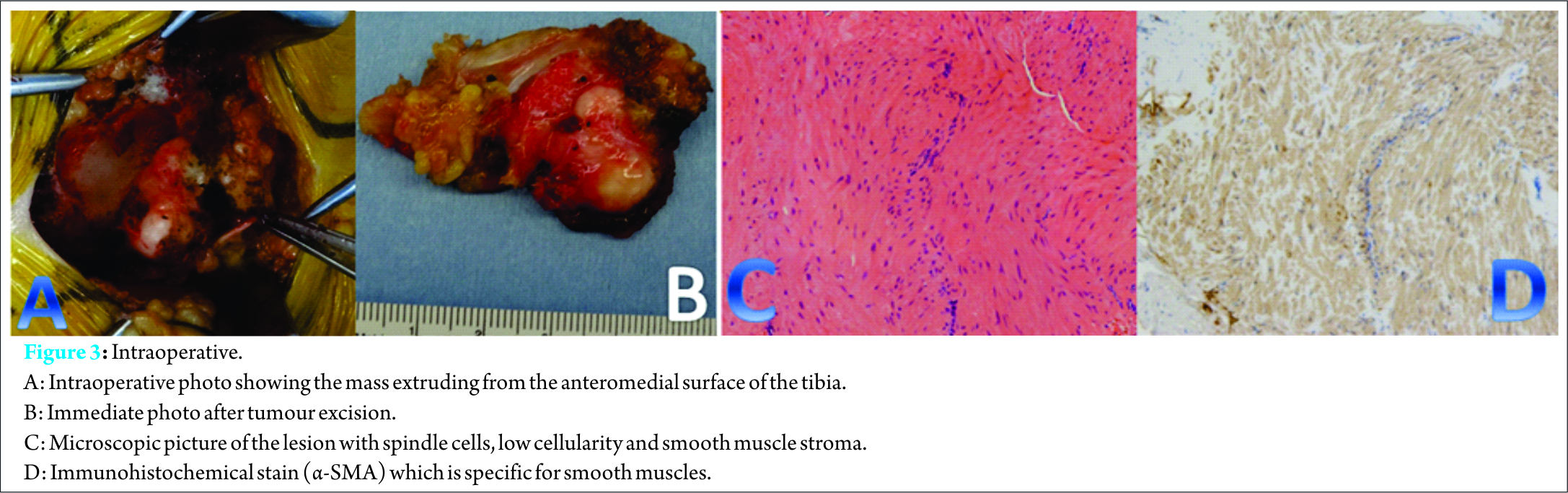
Discussion
Leiomyomata are very rare in the bone. Most of reported intraosseous leiomyomata arise from axial skeleton. First case of intraosseous leiomyoma was in the maxillary tooth socket and it was described in 1976 by Rhatigan and Kim [10]. Loyola et al. revised 11cases involving the mandible and maxilla [11]. The lesion is recorded in other areas of axial skeleton such as skull, spine, as well as a single case of the rib has been reported [12], while only limited cases of intraosseous leiomyomata in peripheral skeleton have been published. In published English literature, eleven cases were found with peripheral skeleton (Limbs and pelvis except sacrum). Average age for the reported cases including our case was 44.5y±10y, 4male representing 33.3% and 8 female representing 66.6%, with range between 37-73years; our case is the oldest, at 73years, among all reported cases of intraosseous leiomyoma. Bone predilection was noticed among the reported cases, as in six out of twelve cases the lesion was in the tibia 50%, two cases of femur 16.6%, two cases in the pelvic bone 16.6%, one case of fibula (8.3%) and ulna (8.3%) each. Another trend that deserves attention is location of tumor within the bone; we noticed that primary intraosseous leiomyomata of peripheral skeleton seem to arise from epi-metaphyseal region, while four cases were in the distal epi-metaphysis, three were in the proximal metaphysis, three cases with periosteal located lesion at diaphyseal level, one case in the pubic bone and another case was in in the iliac crest Table (1). Diagnosis of this tumor as well as of the previous reported cases was not straightforward, it included many differential diagnoses, nopathognomonic clinical sign was related to intraosseous leiomyoma though nonspecific pain is the main presentation of intraosseous leiomyoma. Swelling and difficult weight bearing are also less frequent complaints. Radiographic differential diagnosis includes a variety of benign primary bone tumors, i.e. osteoid osteoma, non-ossifying fibroma, hemangioma, adamantinoma in addition to malignant tumors such as leiomyosarcoma, and metastasis. In osteoid osteoma, there is little sclerosis surrounding a central calcified nidus which is also surrounded by new bone formation. CAT scan clearly reveals the nidus in most cases though it might be easily missed in MRI which usually reveals bone marrow edema. In non-ossifying fibroma, the lesion is typically sharply demarcated, asymmetrical, cortically based lucency with a thin sclerotic rim. It often appears as a multiloculated lesion. It is located in the metaphysis adjacent to the physis. As the patient ages it seems as if it migrates away from the physis, there is no associated periosteal reaction, cortical breach or associated soft tissue mass. On MRI appearances are variable, and depend on when along the development and healing phase the lesion is imaged. Initially the lesion has high or intermediate T2 signal with a peripheral low signal rim corresponding to the sclerotic border as it matures and begins to ossify, the signal becomes low on all sequences, and contrast enhancement is also variable. In surface hemangiomas, it could be associated with cortical thickening, cortical erosion, and a lytic focus resembling a nidus on CT. A soft tissue mass extending from the cortex might be seen but is an infrequent finding [10]. In adamantinoma which was included in differential diagnosis because of the tibial location of the lesion, it appears as a multi-locular or slightly expansible osteolytic lesion. This may be visualized as areas of lysis interspersed with areas of sclerosis. Lesions tend to have an eccentric epicenter and a lack of periosteal reaction. There may be a locally aggressive disease at presentation. On MRI, it might be presented as one of two morphologic patterns either a solitary lobulated focus or multiple small nodules in one or more foci [13]. In leiomyosarcoma, radiological feature depends on grade of malignancy, in high grade leiomyosarcomas there is ill-defined, irregular osteolytic lesion with a moth-eaten or permeative pattern of osseous destruction radiographically, and diffuse involvement and destruction of the medullary bone histologically. On the other hand, low grade tumors exhibit diffuse involvement of the marrow spaces, and have radiographic features, including a geographic pattern of bone destruction and a sclerotic rim [14]. Calcification is exceedingly rare in leiomyosarcoma. In MRI, it may frequently demonstrate cystic foci within. The lesion appears so intense to muscle T1, while inT2 intermediate to hypo intense to neighboring fat, and predominantly hyper intense in T2FS. Metastasis is not a far possibility, it would occur from primary carcinomas, including lung, breast, kidney, and pancreas. The thin capsule seen around the lesion, as well as its oval shape and sharply marginated edge made metastasis less likely. We considered adamantinoma far less likely in the differential diagnosis, as histologically this neoplasm is noted for its heterogeneity and presence of epithelial islands. A malignant spindle cell tumor was not considered because the tumor lacked pleomorphism, high rate of mitoses, and necrosis. The sclerotic margins seen on the conventional radiograph and in the histologic sections also supported the benign nature of this tumor. However, absence of signs of malignancy must be carefully documented in order to rule out leiomyosarcoma.
Histologically; leiomyoma is classified into three histological categories: solid leiomyoma, vascular leiomyoma (or angioleiomyoma), and epithelioid leiomyoma. While angioleiomyoma is the most prevalent type in soft tissues, solid leiomyoma represents the majority of cases of intraosseous leiomyomata [15], it exhibits the same histological features as leiomyoma at other sites. It is composed of a proliferation of spindle-shaped cells arranged in thick intersecting bundles. Mitoses are very rarely seen (low rate of mitosis 0-4per ten high power fields) with moderate cellularity and no necrosis.
Angioleiomyomas are further classified into three histological categories: solid (the most common type in soft tissues), venous, and cavernous [15]. However, all three patterns are often present within the same tumor. Similarly, reported cases of intraosseous angioleiomyoma showed no distinctive histological features compared to soft-tissue angioleiomyomas [15]. In published cases, three cases out of eleven were histologically angioleiomyoma, two were in tibia and one in the iliac bone. The use of immunohistochemical staining was helpful in this case, the tumor cells stained strongly for muscle markers, including smooth muscle actin and desmin, thus confirming this tumor’s smooth muscle origin. The neoplastic cells did not stain for neural, fibrous, or epithelial markers (S-100, CD34, and AE1/3, respectively). The standard treatment for intraosseous leiomyoma is extensive tumor excision with wide margin followed by packing of the cavity with autologous cancellous bone graft [4]. Internal fixation is required when the residualbony defect is large. Complete excision of intraosseous leiomyoma is typically curative with excellent prognosis. Till now, no cases of recurrences have been reported after complete excision [4,5], however, regular follow-up is necessary to observe the risk of recurrence. In only one of the previously reported cases, reconstruction using bone graft alone was not enough, and reconstruction by TKA was performed due to difficulty in preserving the articular cartilage and thinning out of the bone [8]. Otherwise, all known cases were treated either by en-bloc excision, surgical resection or curettage. In addition to bone graft, and in all cases, results were satisfactory to excellent with no recurrence, malignant change, or distant metastases, early recovery and restoration of function. In our case, we performed wide tumor excision, the bone defect was not large enough to necessitate internal fixation. Patient satisfaction after surgery was good, with improvement of pain, and preserved function of the knee. Pain was completely relieved by two months post-operatively. There was no recurrence, malignant change or distant metastases at the latest follow up visits.
Conclusion
Diagnosis of Intraosseous leiomyoma of the extremities is difficult due to extreme rarity of the tumor and absence of pathognomonic radiological signs in X-ray, CAT, or even MRI. The exact diagnosis is only achieved by histopathological examination and with immunohistochemistry stains, which can differentiate it from malignancy, especially from the much less rare leiomyosarcoma. Orthopedic oncologists have to include this rare benign tumor in the differential diagnosis of any intraosseous lesion with gradually worsening and long-standing pain, despite of benign imaging characters. Different histological patterns of leiomyoma do exist, however there is no difference in prognosis or treatment options. Treatment standard includes wide excision with autologous bone graft whenever possible. Internal fixation may be necessary if the bone defect is large or there is thinning out of the cortex which may lead to pathological fracture.
Clinical Message
Intraosseous leiomyoma is a rare tumor; it lacks a pathognomonic radiological sign which makes its diagnosis quite difficult. It is better to include this tumor in the differential diagnosis of painful benign intraosseous lesions.
References
1. Kumar V, Cotran RS, Robbins SL. Robbins basic pathology. 7th ed. Philadelphia: Saunders; 2003.
2. Boyd R, Bhatt B, Mandell G, Saxe A. Leiomyoma of the hand: a case report and review of the literature. J Hand Surg Am 1995;20:24-6.
3. Braun W, Kotter A, Kundel K, Wiedemann M, Wagner T.Intraosseous leiomyoma of the neck of the femur. A case report. IntOrthop1994;18:47–49.
4. Zikria BA, Radevic MR, Jormark SC.Intraosseous leiomyoma of the ulna: a case report. J Bone Joint Surg Am. 2004;86:2522–2525.
5. Lafosse JM, Gomez-Brouchet A. Intraosseous leiomyoma: a report of two cases. Joint Bone Spine 2007;74:389–392.
6. Lau, Sean K. “Intraosseous angioleiomyoma the tibia: A case report.” Pathology-Research and Practice 2014;210(5): 321-324.
7. Sung, Yu-Hsiang, et al. “Intraosseous leiomyoma of the distal femur: a case report and review of literatures.” European Journal of Orthopaedic Surgery & Traumatology 2012;22.1:161-165.
8. Seena C. Aisner MD, Marcia Blacksin MD, Francis Patterson MD, Meera R. Hameed MD A Painful Tibial Mass in a 37-year-old Man. ClinOrthopRelat Res 2008;466:756–759.
9. S Jayakody, K. Young, B. Young, R. Ferch. Serial spread of benign metastasizing leiomyoma to the thoracic spine Journal of Clinical Neuroscience 2011;18:1135–1137.
10. Rhatigan RM, Kim ZE. Leiomyoma arising adjacent to a maxillary tooth socket: an intraosseous leiomyoma presenting as an odontogenic lesion. South Med J 1976; 69:493–494.
11. Loyola AM, Araujo NS, Zanetta-Barbosa D, Mendes VC, Jordao- Silva C, Bittar TO. Intraosseous leiomyoma of the mandible. J Oral Maxillofac Pathol. 1999;87:78–82.
12. Ganyusufoglu AK, Ayalp K, Oztu¨rk C, Sakallioglu U, Ozer O. Intraosseous leiomyoma in a rib. A case report. ActaOrthopBelg 2009;75:561–565.
13. Levine SM, Lambiase RE, Petchprapa CN. Cortical lesions of the tibia: characteristic appearances at conventional radiography. Radiographics 2003; 23 (1):157-77.
14. C.R. Antonescu, R.A. Erlandson, A.G. Huvos, Primary leiomyosarcoma of bone: a clinicopathologic, immunohistochemical, and ultrastructural study of 33 patients and a literature review, Am. J. Surg. Pathol 1997; 21:1281–1294.
15. Liang H, Frederiksen N, Binnie W, Cheng Y. Intraosseous oral leiomyoma:systematic review and report of one case. DentomaxillofacRadiol 2003;32:285e90.
| How to Cite This Article: Abdelaal A, Yamamoto N, Hayashi K, Takeuchi A, Tsuchiya H. Intraosseous Leiomyoma of the Tibia. A Case Report. Journal of Orthopaedic Case Reports 2016 April – June;6(2): 81-85. Available from: https://www.jocr.co.in/wp/2016/04/01/2250-0685-448-fulltext/ |
[Full Text HTML] [Full Text PDF] [XML]
[rate_this_page]
Dear Reader, We are very excited about New Features in JOCR. Please do let us know what you think by Clicking on the Sliding “Feedback Form” button on the <<< left of the page or sending a mail to us at editor.jocr@gmail.com