[box type=”bio”] What to Learn from this Article?[/box]
Always keep an open mind about other sources of sepsis i.e. fungi.
Case Report | Volume 7 | Issue 1 | JOCR January – February 2017 | Page 46-49 | Scott Matthews, Sam Sloan, Daniel McCaffrey, Angel Ruiz. DOI: 10.13107/jocr.2250-0685.682
Authors: Scott Matthews[1], Sam Sloan[2], Daniel McCaffrey[3], Angel Ruiz[3]
[1] Department of Orthopaedics, Locum ST3, General Surgery, Mater Hospital, Belfast, Northern Ireland.
[2] Department of Orthopaedics, Clinical Fellow Spines, Royal Victoria Hospital, Belfast, Northern Ireland.
[3] Department of Orthopaedics, Altnagelvin Hospital, Londonderry, Northern Ireland.
Address of Correspondence
Dr. Scott Matthews,
Orthopaedic SHO CT2 – MB BCh BAO MRCS, Locum ST3, General Surgery, Mater Hospital, Belfast, Northern Ireland.
E-mail: wltrmt@aol.com
Abstract
Introduction: Fungal joint infection can lead to serious consequences for those affected. It can often be a delayed diagnosis due to initial negative organism growth or lack of clinician awareness. Treatment should be early and aggressive to prevent patient morbidity and mortality.
Case Report: We present a case of staphylococcal septic arthritis of the native hip joint with secondary superinfection by Candida albicans in a young patient with no appreciable risk factors. We explain the complexity of a delayed diagnosis and subsequent treatment.
Conclusion: This case highlights important learning points in terms of considering secondary fungal infection in any septic arthritis patient that does not respond to conventional antimicrobial treatment.
Keywords: Septic arthritis, fungal infection, hip.
Introduction
The most common causative organism of septic arthritis is Staphylococcus aureus [1]. Primary fungal etiology is rare and rates of secondary infection are unknown. The diagnosis of fungal infection can often be delayed or neglected due to lack of awareness or negative growth [2]. The delay in diagnosis and appropriate treatment can result in increased morbidity and prolonged hospitalization. More commonly, fungal infection occurs secondary to colonization of patients at risk [3, 4, 5, 6]. We present a rare case of secondary Candida albicans septic arthritis of the native hip joint.
Case Report
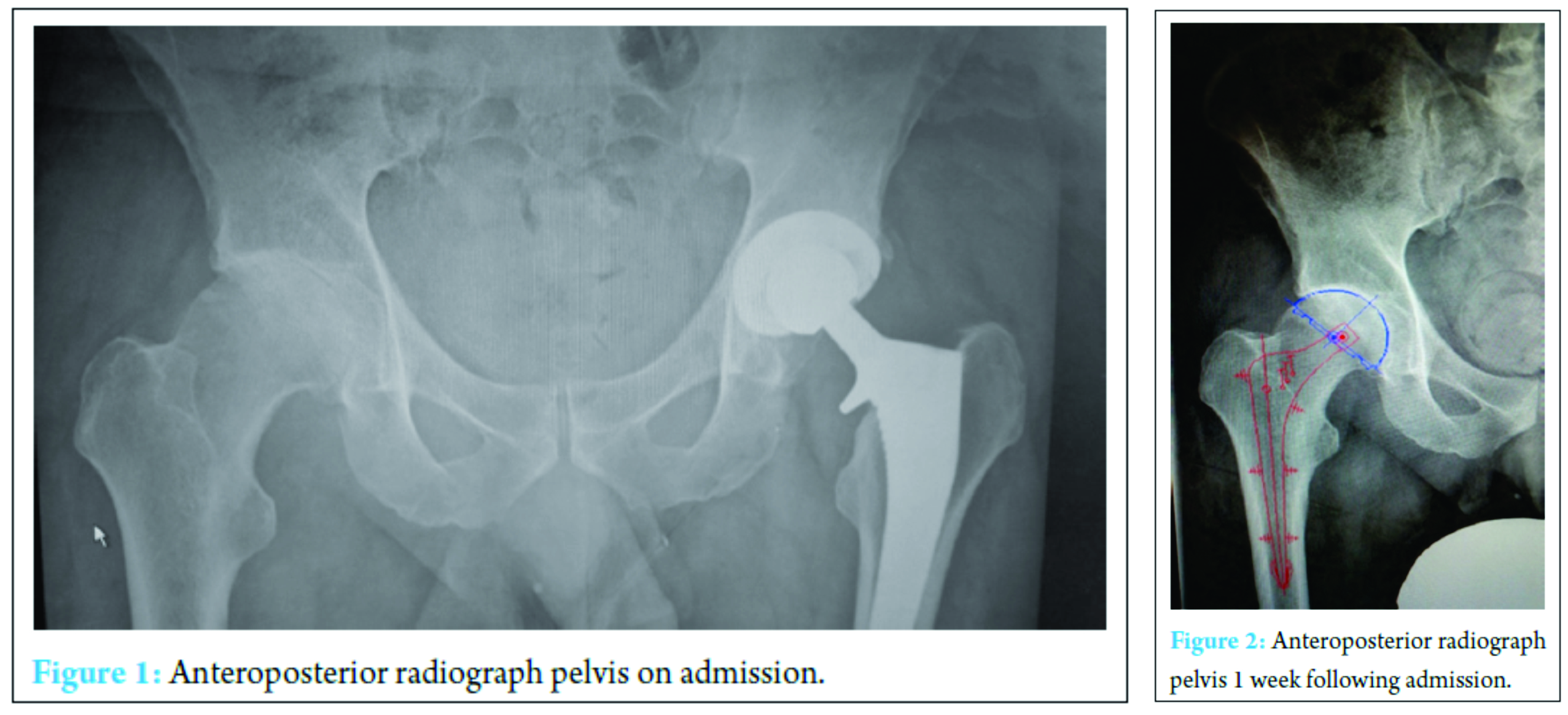
A 59-year-old physical education teacher with known right hip osteoarthritis was admitted with rapid onset of increasing right hip pain, inability to weight bear, pyrexia, and rigors. Relevant medical history included a successful left total hip arthroplasty, hemochromatosis, and hypercholesterolemia. There was no evidence of liver cirrhosis. Blood markers were elevated with a neutrophil leukocytosis of 24 × 103 cells/mm3 and C-reactive protein (CRP) of 514 mg/ml. Normal range 0-5 mg/ml (Fig. 1). Radiographs demonstrated right hip osteoarthritis while an ultrasound scan demonstrated a large echogenic right hip effusion with associated synovial thickening. The patient was managed with an urgent washout and debridement of his right hip joint through an anterior approach. Intraoperatively, intravenous teicoplanin 400 mg was commenced following collection of multiple fluid and tissue samples. A closed suction drain was left in situ that collected 200 ml of serosanguinous fluid in the first 24 h after which the drain was removed. Two pre-operative peripheral blood cultures and four separate fluid and tissue theater samples grew S. aureus, sensitive to flucloxacillin and sodium fusidate. Microbiology advised switching from intravenous teicoplanin 400 mg TID to intravenous flucloxacillin 2 g QDS and oral sodium fusidate 500 mg TID. He initially responded well with falling inflammatory markers, apyrexia, pain reduction, and a dry healing wound (Fig. 2).
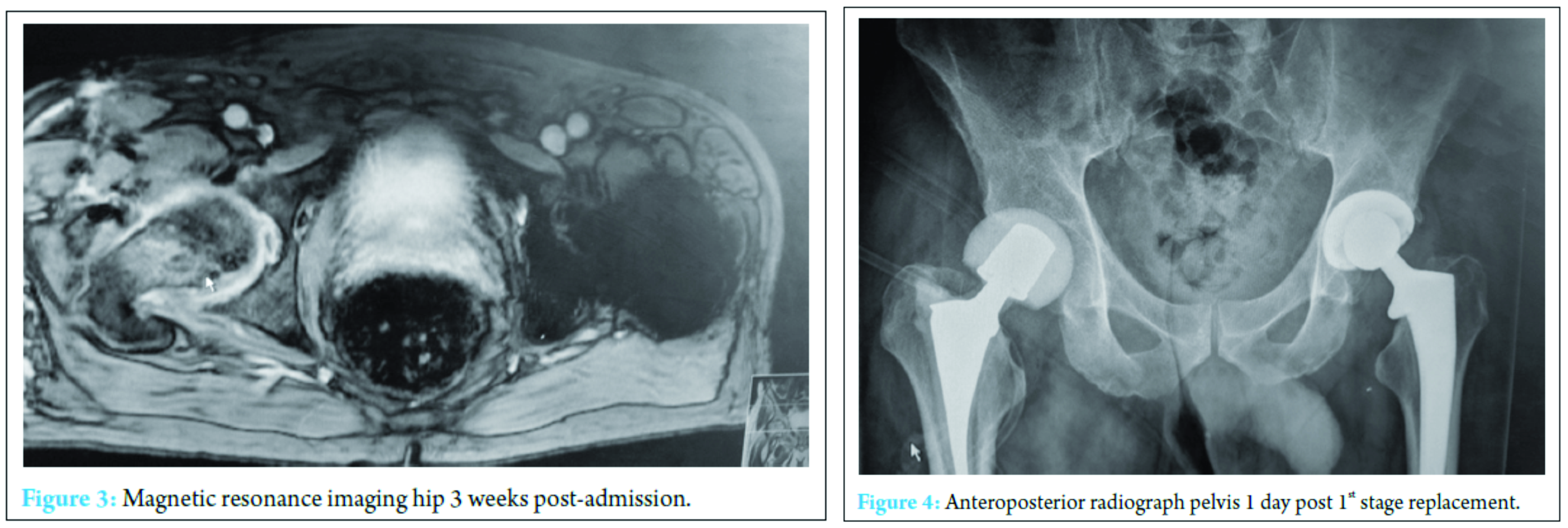
At 2 weeks, the onset of wound ooze and rising markers prompted a repeat washout. Intraoperative samples on this occasion were positive for Pseudomonas aeruginosa and S. aureus prompting the addition of IV piperacillin-tazobactam 4.5 g TID and IV gentamicin 400 mg OD to the IV flucloxacillin and oral sodium fusidate treatment. The patient responded clinically and biochemically with resolution of pain, the absence of pyrexia and a reduction in inflammatory markers to a white cell count of 13 × 103 cells/mm3 and CRP of 41 mg/ml. Despite this positive response, however, the wound ooze persisted (Fig. 3).
A further washout performed at week three revealed a deep fluid collection so the wound was left open and a vacuum-assisted closure (VAC) device applied. Six separate theater specimens were all negative for microbial growth. Following this, the Microbiology Department advised discontinuing IV piperacillin-tazobactam and IV teicoplanin 400 mg TID and meropenem 1 g TID were commenced. Throughout the first few weeks, alternative sources of infection including endocarditis were excluded and a magnetic resonance imaging revealed no pelvic collection or evidence of osteomyelitis. The VAC drainage remained high and further theater wound inspections identified the deep collection was not recurring. One of the deep tissue samples sent in this period was positive for C. albicans. Although initially thought to be a contaminant, oral fluconazole 400 mg OD was commenced given the poor response to polymicrobial therapy. Despite remaining clinically and biochemically well, the wound failed to progress, and therefore a formal first stage excision arthroplasty was performed 8 weeks following admission, with a Biomet stage one select reinforced cement hip spacer impregnated with gentamicin and vancomycin through an anterolateral approach [7] (Fig. 4). Femoral head histopathology confirmed appearances in keeping with osteomyelitis. Throughout the next 2 weeks, four changes of VAC were required to monitor the wound and ensure no deep collection was persisting, due to a pattern of fluctuating inflammatory markers. Positive C. albicans cultures were obtained from only two out of nine deep tissue samples taken intraoperatively despite the patient being on antimicrobial therapy. Peripheral blood cultures remained negative. A diagnosis of secondary fungal septic arthritis was then made given the recurrent positive C. albicans growth from tissue samples. Oral fluconazole 400 mg OD was escalated to intravenous anidulafungin 50 mg OD and antibiotic therapy discontinued following a 10-week course (Fig. 5). He responded well both clinically and biochemically with a white cell count of 10 × 103 cells/mm3 and CRP of 30 mg/ml. The wound settled slowly and progressively thereafter allowing delayed primary closure 9 weeks following admission. Following wound closure, he remained well with a healing wound and therefore was discharged home on his intravenous antifungal treatment. An uncomplicated second stage procedure was performed 3 months following the first stage procedure with implantation of a cemented total hip replacement. Revision copal cement, impregnated with gentamicin and clindamycin as standard was supplemented with 600 mg grams of anidulafungin antifungal powder [8] (Fig. 6).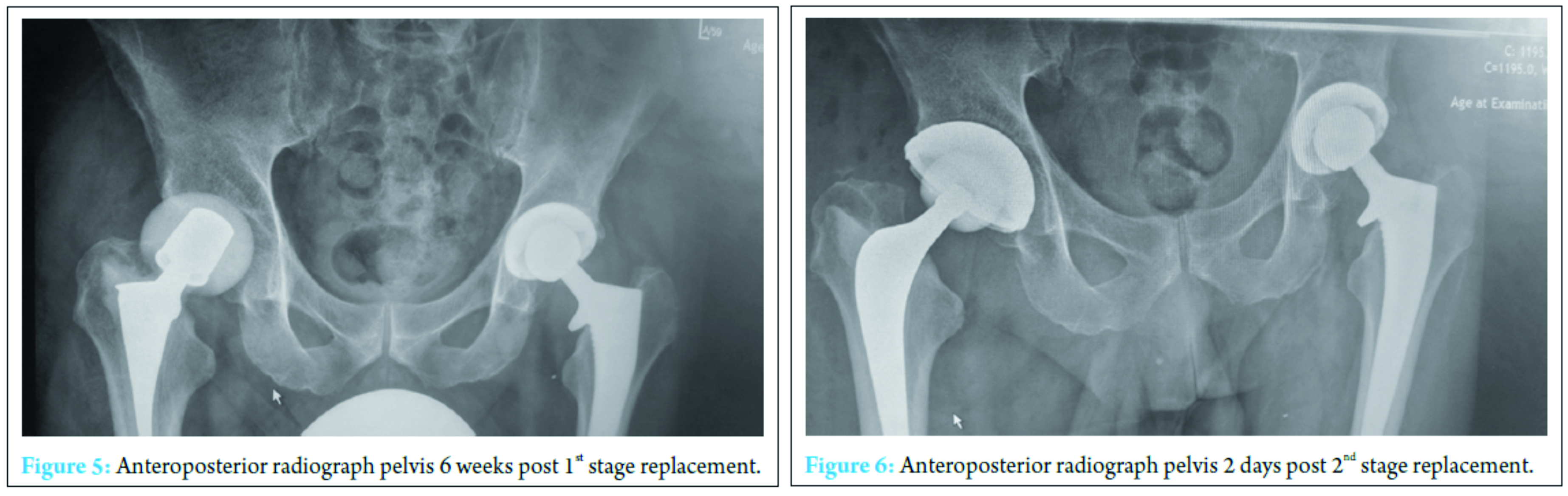
Discussion
Early recognition of septic arthritis is of key importance so appropriate management can be implemented to prevent morbidity. Rapid joint destruction and septic shock are two of the main concerns with delayed diagnosis [9]. Septic arthritis is largely bacterial in etiology however fungal sources are much rarer and quite often overlooked. The most common bacterial etiology includes S. aureus and the most common fungal organism is C. albicans [1, 10, 11]. Fungal septic arthritis can be caused invasively by injection, open operation, or through hematogenous spread commonly in immunosuppressed patients [3]. The main risk factors for fungal septic arthritis include the use of multiple antibiotics, pharmacological immunosupression, diabetes, rheumatoid arthritis, malnutrition, alcohol misuse, and arthroplasty. The presence of these risk factors is becoming increasingly prevalent in society thereby increasing the risk of fungal septic arthritis [3, 4, 5, 6]. Mortality can be as high as 5-15% for septic arthritis [9]. This case was clearly a bacterial septic arthritis at initial presentation however we believe the patient suffered a secondary fungal superinfection due to polymicrobial therapy. Antibiotic therapy can affect normal flora and promote overgrowth of fungal species due to a reduction in the normal levels of colonic microflora [12]. This can result in fungal overgrowth within the colonic mucosa and an imbalance in flora resulting in candidiasis [13]. We presume the etiology of fungal origin in this case is antibiotic induced fungal colonization or as a nosocomial fungal infection following the wound breakdown [3]. The mainstay of any septic arthritis treatment is prolonged antimicrobial therapy in conjunction with joint washout(s) ± debridement. In this instance, we utilized a standard two-stage technique that successfully treated the bacterial and fungal source of sepsis once identified and the appropriate antifungal therapy instituted [1, 5, 9]. The key learning point from this case report is to consider alternative and rare etiological organisms other than bacterial sources when the clinical course deviates from what is expected. The treating clinician should be alerted when patients do not respond to standard antimicrobial treatment for presumed or as in this case confirmed bacterial septic arthritis and as such should consider fungal superinfection as a cause. We believe that this patient was a staphylococcal septic arthritis that was subsequently complicated by with C. albicans following prolonged antibacterial therapy and wound breakdown. The initial wound breakdown may either be a result of failure to respond to the initial washout, i.e., a complication of the bacterial infection, or due to the onset of fungal infection. The fungal superinfection however may have occurred due to colonization of the wound as a result of the open management of the dehiscence. Irrespectively, the fungal superinfection was not detected immediately and therefore resulted in a delay in diagnosis. Failure to culture the fungal pathogen in multiple samples caused a prolonged patient journey, increased morbidity, and increased financial demand on the hospital.
Conclusion
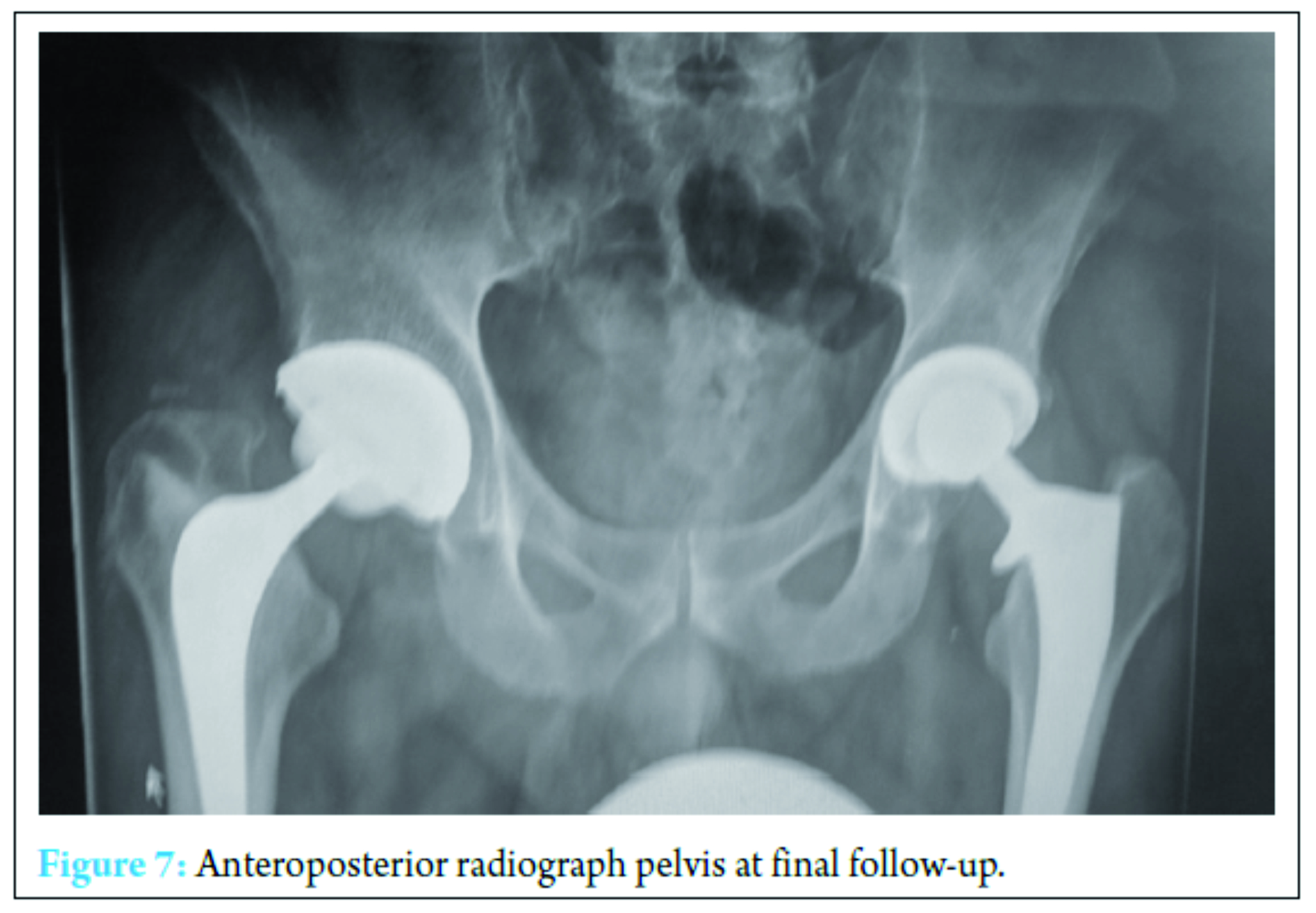
Prompt treatment should be initiated in patients who present with septic joints and potential risk factors should be identified. Fungal etiology should always be considered when treating any septic arthritis and as this case highlighted should be reconsidered if patients do not respond as expected to antibiotic treatment. This case demonstrates that the use of antibiotics may predispose a previously healthy individual to develop a secondary complication of fungal septic arthritis. Early diagnosis helps to reduce the risk of fungal septicemia. Fungal culture can take a long time to become positive and therefore if a small number of samples are positive we would suggest that antifungal therapy is initiated if the patient is not responding with antibiotics [14]. This case report identifies the need for increased awareness of the treating clinician to consider alternative sources of infection.
Clinical Message
Early diagnosis helps to reduce the risk of fungal septicemia. Fungal culture can take a long time to become positive and therefore if a small number of samples are positive we would suggest that antifungal therapy is initiated if the patient is not responding with antibiotics [14]. This case report identifies the need for increased awareness of the treating clinician to consider alternative sources of infection.
References
1. Younkin S, Evarts CM, Steigbigel RT. Candida parapsilosis infection of a total hip-joint replacement: successful reimplantation after treatment with amphotericin B and 5-fluorocytosine. A case report. J Bone Joint Surg Am 1984;66(1):142-143.
2. Kawanabe K, Hayashi H, Miyamoto M, Tamura J, Shimizu M, Nakamura T. Candida septic arthritis of the hip in a young patient without predisposing factors. J Bone Joint Surg Br 2003;85(5):734-735.
3. Arias F, Mata-Essayag S, Landaeta ME, Capriles CH, Pérez C, Núñez MJ, et al. Candida albicans osteomyelitis: Case report and literature review. Int J Infect Dis 2004;8(5):307-314.
4. Favero M, Schiavon F, Riato L, Carraro V, Punzi L. Septic arthritis: A 12 years retrospective study in a rheumatological university clinic. Rheumatismo 2008;60(4):260-277.
5. Marra F, Robbins GM, Masri BA, Duncan C, Wasan KM, Kwong EH, et al. Amphotericin B-loaded bone cement to treat osteomyelitis caused by Candida albicans. Can J Surg 2001;44(5):383-386.
6. Madruga Dias J, Costa MM, Pereira da Silva JA, Viana de Queiroz M. Septic arthritis: patients with or without isolated infectious agents have similar characteristics. Infection 2014;42(2):385-391.
7. Van Thiel GS, Berend KR, Klein GR, Gordon AC, Lombardi AV, Della Valle CJ. Intraoperative molds to create an articulating spacer for the infected knee arthroplasty. Clin Orthop Relat Res 2011;469:994-1001.
8. Ensing GT, van Horn JR, van der Mei HC, Busscher HJ, Neut D. Copal bone cement is more effective in preventing biofilm formation than palacos R-G. Clin Orthop Relat Res 2008;466:1492-8.
9. Ravindran V, Logan I, Bourke BE. Medical vs surgical treatment for the native joint in septic arthritis: a 6-year, single UK academic centre experience. Rheumatology (Oxford) 2009;48(10):1320-1322.
10. Legout L, Assal M, Rohner P, Lew D, Bernard L, Hoffmeyer P. Successful treatment of Candida parapsilosis (Fluconazole-resistant) osteomyelitis with caspofungin in a HIV patient. Scand J Infect Dis 2006;38(8):728-730.
11. Zmierczak H, Goemaere S, Mielants H, Verbruggen G, Veys EM. Candida glabrata arthritis: case report and review of the literature of Candida arthritis. Clin Rheumatol 1999;18(5):406-409.
12. Hickson M. Probiotics in the prevention of antibiotic-associated diarrhoea and Clostridium difficile infection. Therap Adv Gastroenterol 2011;4(3):185-197.
13. Zwolinska-Wcislo M, Brzozowski T, Mach T, Budak A, Trojanowska D, Konturek PC, et al. Are probiotics effective in the treatment of fungal colonization of the gastrointestinal tract? Experimental and clinical studies. J Physiol Pharmacol 2006;57(9):35-49.
14. Pasqualotto AC, Denning DW. Diagnosis of invasive fungal infections – Current limitations of classical and new diagnostic methods. Eur Oncol Rev 2005;1-11. Available from: http://www.touchoncology.com/system/files/private/articles/1289/pdf/onco1552.pdf. [Last accessed on 2016 Mar 21].
| How to Cite This Article: Matthews S, Sloan S, McCaffrey D, Ruiz A. Septic Arthritis of the Hip Complicated by Secondary Fungal Superinfection. Journal of Orthopaedic Case Reports 2017 Jan-Feb;7(1):46-49. Available from: https://www.jocr.co.in/wp/wp-content/uploads/15.-2250-0685.682.pdf |
[Full Text HTML] [Full Text PDF] [XML]
[rate_this_page]
Dear Reader, We are very excited about New Features in JOCR. Please do let us know what you think by Clicking on the Sliding “Feedback Form” button on the <<< left of the page or sending a mail to us at editor.jocr@gmail.com