[box type=”bio”] Learning Point of the Article: [/box]
Staged procedures, angioembolization, characterization as secretary versus non-secretary, and a team approach involving the endocrinologists are the keys to success while dealing with neuroendocrine tumors.
Case Report | Volume 8 | Issue 6 | JOCR November – December 2018 | Page 92-95 | S K Srivastava, Nandan Marathe, Sunil Bhosale, Shaligram Purohit, Aditya Raj, Jitesh Manghwani. DOI: 10.13107/jocr.2250-0685.1276
Authors: S K Srivastava[1], Nandan Marathe[1], Sunil Bhosale[1], Shaligram Purohit[1], Aditya Raj[1], Jitesh Manghwani[1]
[1]Department of Orthopedics, Seth GS Medical College and KEM Hospital, Mumbai, Maharashtra, India.
Address of Correspondence:
Dr. Nandan Marathe,
Saraswati Prasad, Gaul Wada, Vasai (w), Palghar–401201, Maharashtra, India.
E-mail: nandanmarathe88@gmail.com
Abstract
Introduction: Paragangliomas are relatively rare tumors, accounting for only about 0.3% of all neoplasms. Most paragangliomas are defined as benign in nature, but 10–20% possess metastatic potential. There have been scattered reports of metastatic paraganglioma in the literature, but in rare circumstances, paragangliomas can metastasize to the spinal column causing destruction or compression of the spinal cord, clinically manifesting as pain or neurological deficit.
Case Report: We present a case of a 43-year-old man who presented with paresthesia and paraparesis and was found to have pathologic fracture involving D1 vertebra as a manifestation of metastasis from a non-secretory right paratesticular paraganglioma.
Conclusions: We will review the literature on the topic of metastatic paraganglioma, management of paragangliomas involving spine, and touch on the importance of angioembolization, staged procedures, and a team approach.
Keywords: Angioembolization, metastasis, paraganglioma.
Introduction
Paraganglia are clusters of neuroendocrine cells derived from the neural crest and are associated with the sympathetic and parasympathetic nervous systems. Chemodectoma/glomus tumor or paraganglioma is formed when these cells undergo neoplastic transformation. Paragangliomas have an annual incidence of 1/100,000 [1] and account for just 0.3% [2] of all neoplasms making them relatively rare tumors. In the majority of cases, these tumors occur sporadically, but familial forms exist and certain genetic mutations are transmittable to offspring. Adrenal medulla is the most common site for a paraganglioma where it is known as pheochromocytoma, the prototypal sympathetic paraganglioma. Outside the adrenal gland, they typically develop in two general locations: Paravertebral paraganglia and paraganglia in the vicinity of the great vessels of the head and neck. The head and neck region accounts for 70% of the extra-adrenal paragangliomas [3]; most commonly as a carotid body tumor, the prototypical parasympathetic paraganglioma. Paragangliomas usually arise in the fifth or sixth decade of life and present as painless slow-growing masses. Recurrence occurs when the surgical resection is incomplete and half of these tumors prove fatal due to infiltrative growth. Although usually characterized as benign, 10–20% of paragangliomas possess metastatic potential [4, 5]. There have been scattered reports of metastatic paraganglioma in the literature, but rarely do such tumors metastasize to the spine [6, 7].
Case Report
43 years’ male presented with paresthesia and paraparesis for 5 months. The patient had Grade 5 power in bilateral upper limbs, Grade 0 power in bilateral hip and knee, and Grade 3 power in the ankle and toes, with hyperreflexia in deep tendon reflexes with ankle clonus bilaterally. The patient did not have any sensory deficit or bladder bowel involvement. Magnetic resonance imaging (MRI)of the dorsolumbar spine revealedpathologicfractureinvolvingD1vertebra(Fig. 1). Computed tomography (CT)-guided biopsy revealed a highly vascular tumor with cells arranged in nests formed by vascular channels(immunohistochemistry [IHC]: synaptophysin and S100: strongly positive), suggestive of a paraganglioma. Plasma-free normetanephrine was normal (156 pg/ml [0–196]). For the localization of primary tumor 18-F fluorodeoxy glucose- positron emission tomography-CT scan was done which revealed the primary lesion in the right testis(Fig. 2).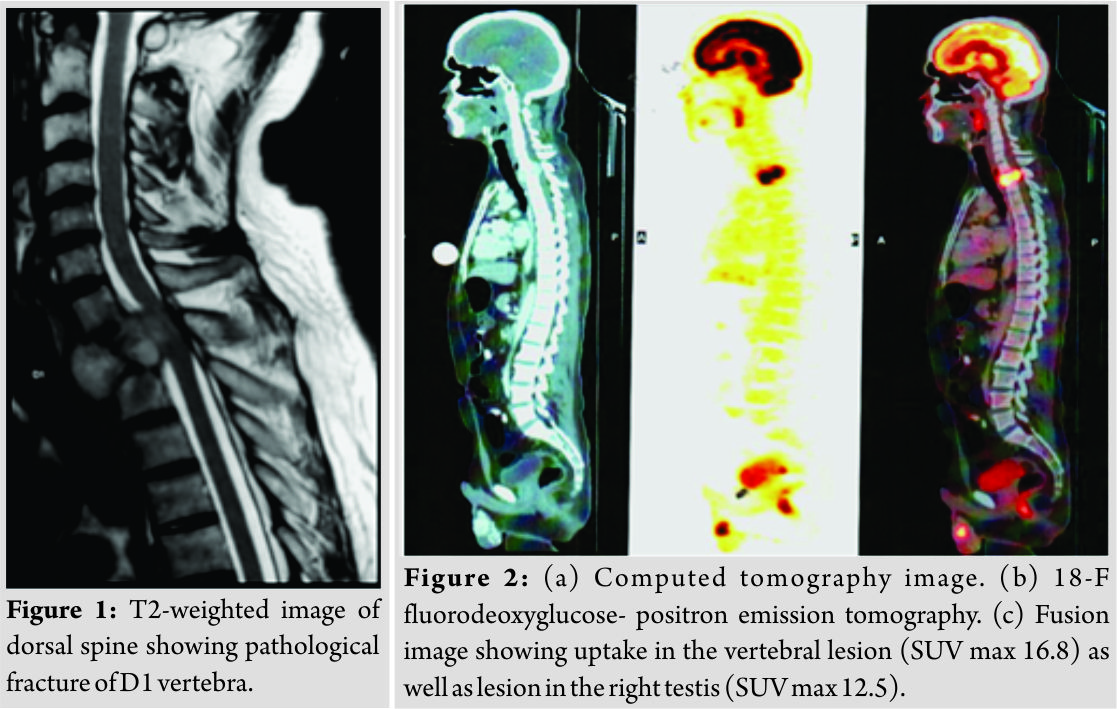
Discussion
The histogenesis of paratesticular paragangliomas has been debated. During infancy, paraganglia have been observed in the paratesticular tissue around the epididymis and spermatic cord [8]. Furthermore, the route of descent of the testis into the scrotum rarely contains heterotopic adrenal glands with medullae; however, both these tissues involute during childhood and persistent tissue has the potential to harbor a neoplasm with potential for malignant spread and may be associated with germ line mutations [9]. It has been cited that there are only about 90 cases in the current literature. Of those cases, most are primary tumors arising from the spine and a minority are paraganglioma that metastasized to the spine. Most patients with spinal paragangliomas are asymptomatic. When symptomatic most present with pain or neurological deficit. Fluctuations in the blood pressure, anxiety, palpitations, diaphoresis, and headache are symptoms associated with functional paragangliomas due to the release of high concentrations of catecholamines. Contrast-enhanced MRI is the diagnostic imaging of choice for paragangliomas. Imaging findings remain relatively non-specific and the diagnosis of paraganglioma depends on histopathological examination. Grossly, paragangliomas are red-pink to brown in color. Microscopically, paragangliomas are mainly composed of nests (Zellballen) of round-to-oval chief cells surrounded by delicate septae composed of sustentacular cells, with minimal exhibition of pleomorphism and mitotic activity. Immunological stains for chromogranin A, synaptophysin, or S100 protein can be targeted for further identification and characterization. The presence of necrosis, mitotic activity, and vascular invasion are suggestive of malignant potential; however, these criteria are not well defined. As evidenced in our patient, the histological morphology and immunohistochemical profile were consistent with these descriptions. Most paragangliomas occur sporadically. However, some are associated with familial syndromes such as Von Hippel-Lindau disease, multiple endocrine neoplasia type 2, neurofibromatosis type 1, or Carney’s syndrome. About 35% of the paragangliomas involving the spine are metastatic lesions. However, it has been reported that it is almost impossible to determine histologically which paraganglioma tumors will demonstrate malignancy in their future clinical course; mitoses, pleomorphism, and vascular invasion have not shown to be reliable indicators. There is ongoing debate as how to best determine malignant potential in paragangliomas, but most are not officially diagnosed until frank metastasis occurs. The mainstay treatment of paragangliomas is surgical and is especially important in paragangliomas involving the spine, as these tumors can cause significant mass effect, leading to pain or neurological deficit. Two additional precautions need to be taken into account preoperatively when considering resection of paragangliomas; functionality and vascularity of the tumor. Paragangliomas can be subdivided into functional or non-functional categories, based on the tumor’s ability to secrete catecholamines or other hormonal substances at a concentration that causes clinically evident symptoms. Paragangliomas that originate from parasympathetic tissue are usually non-functional, but tumors of sympathetic origin often are functional and precaution needs to be taken before resection because manipulation of such tumors can lead to catecholamine leak or surges causing hemodynamic instability and cardiovascular collapse. To prevent this, it has been recommended that alpha- and beta-blockade should be implemented 2 weeks before surgery to minimize morbidity and mortality in functional tumors [10]. The second concern is the hypervascular character of paragangliomas, which can lead to significant blood loss intraoperatively. This is consistent with the report by Capella et al. [3] in which their patient underwent partial corpectomy and had significant blood loss of 3000 cc requiring considerable resuscitation intraoperatively. It has been suggested that pre-operative embolization of the tumor 24–48hours before surgery can minimize blood loss when treating paragangliomas of the spine [11]. Our patient underwent angioembolization 24 hours before the second stage of the surgery. Total resection should be the goal, as these tumors have a reported high rate of recurrence when partially resected. Richter et al. [12] demonstrated that total en bloc spondylectomy complemented with radiotherapy was able to provide tumor-free survival at 10-year follow-up in their patient. However, given the intricate growth pattern and encasement of neural tissue by paraganglioma tumors and the proximity of the spine to other vital structures, total resection is not always possible. Nonetheless, maximal resection should be attempted. Although there is a paucity of studies that directly examine the role of chemotherapy and radiotherapy for spinal paragangliomas, such adjuvant modalities are advocated. It is recommended that a total of 45Gy [10] be given for paraganglioma lesions involving the spine. Some studies suggest that octreotide and other somatostatin analogs are effective complementary treatments for paragangliomas. In cases where surgical resection is not an option, combination of radiotherapy and systemic anticancer octreotide therapy can be relatively effective for paragangliomas involving the spine. U-King-Imet al. [13] described a case in which a 32-year-old female presented with vertebral metastatic paraganglioma that was successfully treated without surgery using a combination of radiotherapy and octreotide; the patient had significant neurological improvement and no progression of disease at her 3-year follow-up visit.
Conclusion
Metastatic paraganglioma involving the spine is a rare entity and management of such cases can be difficult. When possible, en bloc resection of the tumor is the goal of surgery. Adjuvant therapy in the form of chemo/radiotherapy can be added if the margins are not adequate to reduce the recurrence rate.
Clinical Message
Staged procedures, angioembolization, characterization as secretary versus non-secretary, and a team approach involving the endocrinologists are the keys to success while dealing with neuroendocrine tumors.
References
1. McNichol AM. Differential diagnosis of pheochromocytomas and paragangliomas. EndocrPathol2001;12:407-15.
2. Lehmen JA, Babbel DM, Mikhitarian K, Choma TJ. Paraganglioma presenting as metastatic lesion in a cervical vertebra: A case report and review of the literature. Spine (Phila Pa 1976) 2010;35:E152-4.
3. Capella C, Riva C, Cornaggia M, Chiaravalli AM, Frigerio B, Solcia E, et al.Histopathology, cytology and cytochemistry of pheochromocytomas and paragangliomas including chemodectomas. Pathol Res Pract1988;183:176-87.
4. Andersen KF, Altaf R, Krarup-Hansen A, Kromann-Andersen B, Horn T, Christensen NJ, et al. Malignant pheochromocytomas and paragangliomas – the importance of a multidisciplinary approach. Cancer Treat Rev 2011;37:111-9.
5. Osborn RE, Mojtahedi S. Paraganglioma metastatic to the cervical spine. ComputRadiol1986;10:167-70.
6. Brodkey JA, Brodkey JS, Watridge CB. Metastatic paraganglioma causing spinal cord compression. Spine (Phila Pa 1976) 1995;20:367-72.
7. Jack EA, Sim JD. A case of paraganglioma with metastasis to the spine. Br J Surg 1953;41:103-5.
8. Garaffa G, Muneer A, Freeman A, Abdel Raheem AM, Ralph DJ, Minhas S, et al. Paraganglioma of the spermatic cord: Case report and review of the literature. ScientificWorldJournal2008;8:1256-8.
9. Alataki D, Triantafyllidis A, Gaal J, Rodiou C, Vouros J, Papathanasiou A, et al. A non-catecholamine-producing sympathetic paraganglioma of the spermatic cord: The importance of performing candidate gene mutation analysis. Virchows Arch 2010;457:619-22.
10. Simpson LN, Hughes BD, Karikari IO, Mehta AI, Hodges TR, Cummings TJ, et al. Catecholamine-secreting paraganglioma of the thoracic spinal column: Report of an unusual case and review of the literature. Neurosurgery 2012;70:E1049-52.
11. Kwan RB, Erasmus AM, Hunn AW, Dubey A, Waites P, Jessup PJ, et al. Pre-operative embolisation of metastatic paraganglioma of the thoracic spine. J Clin Neurosci2010;17:394-6.
12. Richter A, Halm HF, Lerner T, Liljenqvist UR, Quante M. Long-term follow-up after en bloc resection and reconstruction of a solitary paraganglioma metastasis in the first lumbar vertebral body: A case report. J Med Case Rep 2011;5:45.
13. U-King-Im JM, Carroll TA, Morris K. Vertebral metastatic chemodectoma: Imaging and therapeutic octreotide. Case report. J Neurosurg 2002;97:106-9.
 |
 |
 |
 |
 |
| Dr. S K Srivastava | Dr. Nandan Marathe | Dr. Sunil Bhosale | Dr. Shaligram Purohit | Dr. Jitesh Manghwani |
| How to Cite This Article: Srivastava S K, Marathe N, Bhosale S, Purohit S, Raj A, Manghwani J. Paratesticular Paraganglioma with Metastasis to D1 Vertebra- A Case Report and Review of Literature. Journal of Orthopaedic Case Reports 2018 Nov-Dec; 8(6): 92-95. |
[Full Text HTML] [Full Text PDF] [XML]
[rate_this_page]
Dear Reader, We are very excited about New Features in JOCR. Please do let us know what you think by Clicking on the Sliding “Feedback Form” button on the <<< left of the page or sending a mail to us at editor.jocr@gmail.com





