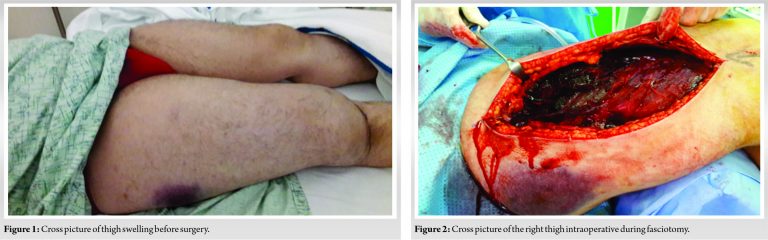
Possibility of acquired factore VIII deficiency should be raised in case of spontaneous atraumatic compartment syndrome.
Dr. Raed Y Abudaqqa,
Department of Orthopaedic, Al Khor, Hospital, Hamad Medical Corporation, Doha, Qatar. E-mail: rabudaqqa@hamad.qa
Introduction: We present a case of spontaneous compartment syndrome due to a very rare cause which is acquired hemophilia.
Case Presentation:A 34-year-old patient presented with the right thigh swelling and features of acute compartment syndrome without history of trauma. He had no history of bleeding disorder. There were no features of infection. As his initial blood tests were within normal 16 g/dl, and his compartment syndrome worsened, he had decompression of the thigh. During the post-operative period, the patient developed persistent bleeding from the decompressed wounds and a fall in hemoglobin which led to further investigation when the blood profile showed a deficiency of factor VIII and antibodies to factor VIII, diagnosis of acquired hemophilia was made and appropriate treatment given.
Conclusion: If atraumatic compartment syndrome diagnosed, possibility of acquired factor VIII deficiency should be raised by isolated prolonged activated partial thromboplastin time and diagnosis confirmed by measuring factor VIII activity level and detecting any factor’s VIII antibodies in blood, such as in this case, the factor VIII level was 5.5 (very low) and against factor VIII antibodies level was 60.8 (high). Here, hematologist should be involved in management.
Keywords: Compartment syndrome, atraumatic, acquired factor VIII deficiency.
Acute compartment syndrome is an emergency that requires a quick treatment, acute compartment syndrome defines as increased intercompartment pressure of any muscle group, this can cause reversible or irreversible muscle or limb damage. Acute compartment syndrome in orthopedic practice is common after high-energy trauma was bleeding from fractures or torn blood vessels into closed compartment of upper or lower limb causes increased compartmental pressure. Crush injuries, burns, and infections can also cause delayed compartment syndromes. Compartment syndrome may be acute or chronic but the acute type is relatively common, on the other hand, chronic exertional compartment syndrome is usually induced by exercise which causing pain and swelling of the affected limb. Exertional chronic compartment syndrome is common in athletes, young adult, and runners who’s exposed to heavy exercise. This chronic compartment syndrome often occurs bilaterally on the lower limb, and manifesting with aching, cramping, or burning pain, tightness, numbness, tingling, or weakness on the affected limb, the character of pain of chronic exertional compartment syndrome is exercise related. Acquired hemophilia causing spontaneous compartment syndrome is very rare and this is case reported in this case study. In these cases, diagnosis of the bleeding disorder is missed as the patient has no history of bleeding and routine tests such as bleeding time and clotting time platelets and INR are normal. Acquired hemophilia A also termed acquired inhibitors against factor VIII, it occurs very rarely in unknown hemophilic population, according many articles [1], incidence about 1–4 per million/year. Although its uncommon cases, these are associated with a high rate of mortality and morbidity as rapid acute incidence, severe bleeding occurs in most of cases up to 90%, and the mortality rate high about 8%–22%. Hence, patients with acquired hemophilia are a clinical challenge. The incidence of acquired hemophilia A is being a very rare in children and more with old age, incidence in children below 16 years about 0.045 per million/year, on the other hand, 14.7 per million/years in the elderly aged above 85 years. Early diagnosis of the condition would allow one to correct the deficiency of factor VIII, before decompression, giving priority to saving life over saving limb and avoid unnecessary morbidity.
A case of 34-year-old male who presented to the emergency department with spontaneous onset of pain and swelling of the thigh of 12 h duration. The pain started 12 h ago when he was treated in private hospital with analgesia and as the pain did not subside and swelling of the thigh increased, he came to the emergency department. He had no history of trauma, prolonged exercises before onset of pain. There was no history of bleeding disorders in the past. Three weeks earlier, he had a history of renal colic for which he took analgesia. This patient worked as automobile electrician.
He was assessed by the emergency physician and the following investigation was done. Hemoglobin 16 g/dl, platelets normal, bleeding time and clotting time normal, electrolytes and liver function normal. X-ray of the hip and thigh was normal and ultrasound of the thigh showed no deep vein thrombosis but diffuse thickening and edema of the thigh. Orthopedic consult was done as the thigh swelling was increasing and the patient was having intractable pain and inability to flex the knee and dorsiflex his foot. The dorsalis pedis and posterior tibial pulses on the right side were weak compared to the left and he had mild subcutaneous bruising of the lateral aspect of thigh. A CT angiogram was ordered which was reported showed diffuse myositis which could be due to infection, inflammatory myopathy with no localized hematoma. An MRI of the thigh was reported as picture of diffuse myositis possibly hemorrhagic involving anterior and lateral compartment muscles of thigh with intramuscular hematoma involving rectus femoris and obliteration of intermuscular fat planes with associated edematous and fluid signal changes along same planes as described above. As the swelling was increasing and pain was not subsiding even strong narcotics and clinical features of compartment syndrome and the pulse of lower limb getting weak, it was decided to decompress the thigh compartment which was done under general anesthesia. The wound was left open and the thigh compartment muscles looked dusky. There was generalized ooze from the wound and few pockets of hematoma is fascial planes. Twelve hours after decompression blood investigation showed a drop in hemoglobin from 16 to 7 g/dl and then to 4.8 g/dl. The patient was started on blood transfusion. The hematologist was consulted and further investigation was done which revealed a very low factor VIII (5.5) very low and antibodies against factor VIII were (60.8) very high.
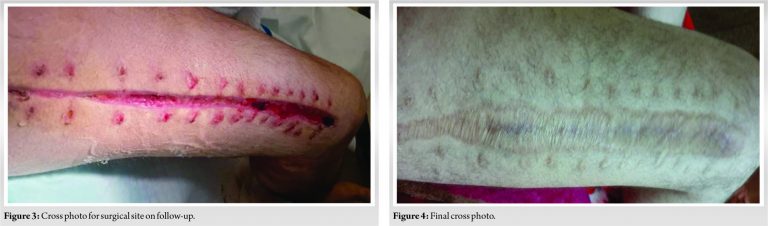
He was transfused about 22 units of packed cells/18 units of fresh frozen plasma, cryoprecipitation 16 units, 6 units platelets, factor VII, prednisolone, cyclophosphamide, and rituximab. Twenty-four hours after decompression, the oozing subsided and the patient was taken for secondary closure of the wound. He made an uneventful post-operative recovery and discharged home.
Acute compartment syndrome is orthopedic emergency which in the lower limbs occurs mostly below knee. Thigh compartment syndromes are rare as its larger compartment space and more complaint and can accommodate expanding hematoma [1]. If the intra-compartmental pressure increases and exceeds the perfusion pressure, the microcirculation becomes oppressed and the tissue viability is jeopardized. If left untreated, it causes irreversible limb ischemia or systemic complications (renal insufficiency, even death) most of compartment syndrome due to limb trauma, fracture, burn, infection, and vascular issues, however very rare to causes other than the previous ones [3, 4]. Bleeding disorder, especially acquired hemophilia A, is very rare causes of spontaneous compartment syndrome. The incidence of acquired hemophilia A is 1–4 per million/year, which is very rare [5]. This condition mostly due to autoantibodies against factor VIII, and most of these cases associated with autoimmune, rheumatological disorders, malignancy, drug reaction, pregnancy, and infection, whereas idiopathic causes reported more on older age patient above 50 years. In comparison with congenital factor VIII deficiency, it has been reported to be as higher mortality morbidity ratio. A case series published by Sallah et al. [11] who reported eight cases of anti-factor VIII inhibitors associated with hematologic malignancy (three cases chronic lymphocytic leukemia, one case acute myeloid leukemia, one case multiple myeloma, one case myelofibrosis, one case lymphoma, and one case myelodysplastic syndrome). Two of these patients did not respond to treatment and died due to major hemorrhage. Author suggested different pathogenesis explaining this association, including disturbances in the antigen presenting cell process, changes of the factor VIII molecule, and changes in the function/interaction of B and T cells. Acquired factor VIII deficiency disease shows mainly soft-tissue bleeding such as muscle, skin, and mucous membrane, however, congenital hemophilia A typically manifests with hemarthrosis. In the majority of the patients, bleeding events precipitated a diagnosis, the pattern of bleeding is differing from congenital hemophilia and not necessarily depending on the residual activity of factor VIII, its evidenced by severe bleeding in some patients although in the presence of relatively high factor VIII levels. Usually, bleeding events occurred spontaneously, less than 10% caused by trauma or surgical procedures. About 4.5% of the patients dying from bleeding [12]. According to Franchini and Lippi [4] for the treatment of cute hemorrhage suggested two options for control of acute bleeding: First, using of bypassing agents and second by aiming to raise the level of factor VIII in circulation. Selecting the most suitable option supposed to be depend on the severity and site of bleeding and titer of factor VIII inhibitor [4, 13] with notice, to avoid any triggering effect or risks of additional bleeding such as invasive procedures, intramuscular injections or the use of exaggerating medications. In the current case, the challenge is the acquired hemophilia was diagnosed only during the second surgery due to ongoing hemorrhage. According to Dumontier et al., if the coagulation disorder can be diagnosed early in the patient’s presentation, surgery should be considered only if no improvement has been shown within 24 h after initiation of replacement therapy and immobilization. Management of acute bleeding disorders with bypassing agents, either activated prothrombin complex or recombinant factor VIIa, and antibody eradication with steroid alone or combined with immunosuppressive medications such as intravenous immunoglobulin, cyclophosphamide, or rituximab. To get better efficacy and economic benefits of acquired hemophilia therapy with bypassing agents, extracorporeal devices or factor concentrates need the specialized team as soon as a patient has been diagnosed, treatment should be guided by expert team in the management of this disorder [12].
If atraumatic compartment syndrome diagnosed, possibility of acquired factor VIII deficiency should be raised by isolated prolonged activated partial thromboplastin time and diagnosis confirmed by measuring factor VIII activity level and detecting any factor’s VIII antibodies in blood, such as in this case, the factor VIII level was 5.5 (very low) and against factor VIII antibodies level was 60.8 (high). Here, hematologist should be involved in management.
The acquired factor VIII should be considered in all patients with atraumatic compartment syndrome, thus avoiding unnecessary morbidity. As specific test is required for testing antibodies to factor VIII and measurement of factor VIII levels which are not done in routine testing of coagulopathy, a high index suspicion is required to make a diagnosis of this condition.
References
- 1.Franchini M, Lippi G. Acquired factor VIII inhibitors. Blood 2008;112:250-5. [Google Scholar]
- 2.Kakkar R, Ellis M, Fearon PV. Compartment syndrome of the thigh as a complication of anticoagulant therapy in a patient with a left ventricular assist device (Berlin Heart). Gen Thorac Cardiovasc Surg 2010;58:477-9. [Google Scholar]
- 3.Matsen FA, Winquist RA, Krugmire RB Jr. Diagnosis and management of compartmental syndromes. J Bone Joint Surg Am 1980;62:286-91. [Google Scholar]
- 4.Franchini M, Lippi G. Acquired factor VIII inhibitors. Blood 2008;112:250-5. [Google Scholar]
- 5.Hoyer LW, Gawryl MS, de la Fuente B. Immunochemical characterization of factor VIII inhibitors. Prog Clin Biol Res 1984;150:73-85. [Google Scholar]
- 6.Collins P, Macartney N, Davies R, Lees S, Giddings J, Majer R. A population based, unselected, consecutive cohort of patients with acquired haemophilia A. Br J Haematol 2004;124:86-90. [Google Scholar]
- 7.Green D, Lechner K. A survey of 215 non-hemophilic patients with inhibitors to Factor VIII. Thromb Haemost 1981;45:200-3. [Google Scholar]
- 8.Scott-Timperley LJ, Haire WD. Autoimmune coagulation disorders. Rheum Dis Clin North Am 1997;23:411-23. [Google Scholar]
- 9.Dumontier C, Sautet A, Man M, Bennani M, Apoil A. Entrapment and compartment syndromes of the upper limb in haemophilia. J Hand Surg Br 1994;19:427-9. [Google Scholar]
- 10.Available from: http://www.orthobullets.com/trauma/1063/thigh-compartment-syndrome. [Google Scholar]
- 11.Franchini M, Targher G, Manzato F, Lippi G. Acquired factor VIII inhibitors in oncohematology: A systematic review. Crit Rev Oncol Hematol 2008;66:194-9. [Google Scholar]
- 12.Demographic and Clinical Data in Acquired Hemophilia A: Results from the European Acquired Haemophilia Registry (EACH2). Available from: https://www.uptodate.com/contents/acquired-inhibitors-of-coagulation/abstract/23. [Google Scholar]
- 13.Green D. The management of acquired haemophilia. Haemophilia 2006;12:2-6. [Google Scholar]
- 14.Mayo Clinic. Chronic Exertional Compartment Syndrome-Symptoms and causes. United States: Mayo Clinic; 2021 [Google Scholar]
- 15.Patient Education: Acute Compartment Syndrome (The Basics). United States: UpToDate; 2021. [Google Scholar]