The use of ibandronate as an adjuvant to core decompression in osteonecrosis femoral head is a cheaper alternative showing promise in short term results.
Dr. Karan Jindal, Department of Orthopaedics Postgraduate Insti-tute of Medical Education and Research, Chandigarh, India. E-mail: karan.121@hotmail.com
Background:Core decompression (CD) is an effective method in the management of pre-collapse stage of osteonecrosis of the femoral head (ONFH). It has been combined with various adjuvants to increase the efficacy including bone marrow aspirate concentration and platelet rich plasma. We evaluated a cheaper alternative in the form of ibandronate as an adjuvant to CD and to highlight the technique, safety, and early outcomes.
Methods:The patients in the age group of 18–60 presenting with atraumatic ONFH in the pre-collapse stage were included in the study. The patients were followed up to a period of 1 year and evaluated in terms of radiologic progression and functional outcome (Modified Harris Hip Score [MHHS] and Visual Analog Scale [1]).
Results:The study included six hips in five patients (three females and two males) ranging from 18 to 57 years. There was no sign of stage progression or collapse on radiography. The average post-operative MHHS was 87.4 (73.7-96.7) while VAS score improved from 2.8 to 1.3 postoperatively (p=0.00395).
Conclusion: The use of ibandronate as an adjuvant with CD shows promise. It is safe and provides good clinical improvement and is a cheaper alternative. However, the interpretation is limited due to the small number of patients and short duration of follow-up.
Keywords:osteonecrosis, ibandronate, outcomes, safety, intraosseous.
Osteonecrosis of femoral head (ONFH) is a progressive degenerative disease characterized by interruption of subchondral bone blood supply, which results in osseous necrosis, diminished support to the articular cartilage and its subsequent collapse. If not treated in time, it culminates in femoral head distortion and secondary osteoarthritis [1]. Disease progression in femoral head ONFH has been shown to correlate with progressively worsening functional outcomes and advanced ONFH is among the most common indications for total hip arthroplasty [2].
The treatment of ONFH varies by the stage of presentation. In pre-collapse stages where the articular cartilage is intact, the treatment modalities such as non-operative management in the form of symptomatic management and oral bisphosphonates or surgical interventions such as core decompression (CD), osteotomies, and bone grafting are used [3, 4, 5]. CD is perhaps the most common surgical intervention, which has been reported to be effective in up to 95% of Ficat Arlet Stage I cases. However, with increased area of necrosis and advanced stage, its efficacy decreases [3, 4, 5, 6]. Methods have been developed to increase the success of the surgery by utilizing adjuvants along with CD; bone marrow aspirate concentrate, platelet rich plasma, and bone morphogenic proteins have shown improved outcomes in randomized studies and systematic reviews [7, 8, 9]. However, these adjuvants are expensive and the cost constraints and regulatory restrictions limit their extensive usage, especially in developing countries. Therefore, there is a need for cost-effective alternatives, which could prove efficacious as adjuvants to CD specially in early ONFH (i.e., modified Ficat and Arlet Stages IIa and IIb [crescent sign]) [10].
Ibandronate is a potent nitrogen-containing bisphosphonate which inhibits farnesyl pyrophosphate synthase, a crucial enzyme in the mevalonic acid pathway essential for cholesterol and isoprenoid lipids synthesis [11]. These lipids are crucial for isoprenylation of proteins needed for osteoclast cellular activities; ibandronate effectively inhibits the osteoclasts activity by inducing apoptosis [12]. Bisphosphonates have been studied in early ONFH through the oral and intravenous routes, and have demonstrated efficacy in halting disease progression [13, 14]. However, complications such as decreased bone turnover, resulting in atypical fractures and jaw necrosis have been re-ported with oral and systemic use [15]. Therefore, topical ibandronate therapy which could have the benefit of maximizing the drug action at the site of ONFH while minimizing systemic complications seems to be an attractive alternative.
Moreover, intraosseous ibandronate has been demonstrated to possess optimal bioavailability and promising results in animal models of ONFH [16, 17].
Hence, we conducted a feasibility study to determine the efficacy and safety of a novel treatment combination of CD and intraosseous ibandronate in pre-collapse stages of ONFH.
This pilot study was initiated after Institutional Ethic Committee clearance vide no. INT/IEC/2020/SPL-936. This present paper is a part of an ongoing trial registered in CTRI (CTRI/2020/09/028112). The patients between age group 18 and 60 years, presenting with atrau-matic ONFH till the crescent stage, were recruited in the study after written informed consent. Traumatic and post-collapse stages with femoral head distortion or osteoarthritis were excluded from the study. Five consecutive patients were included in the study.
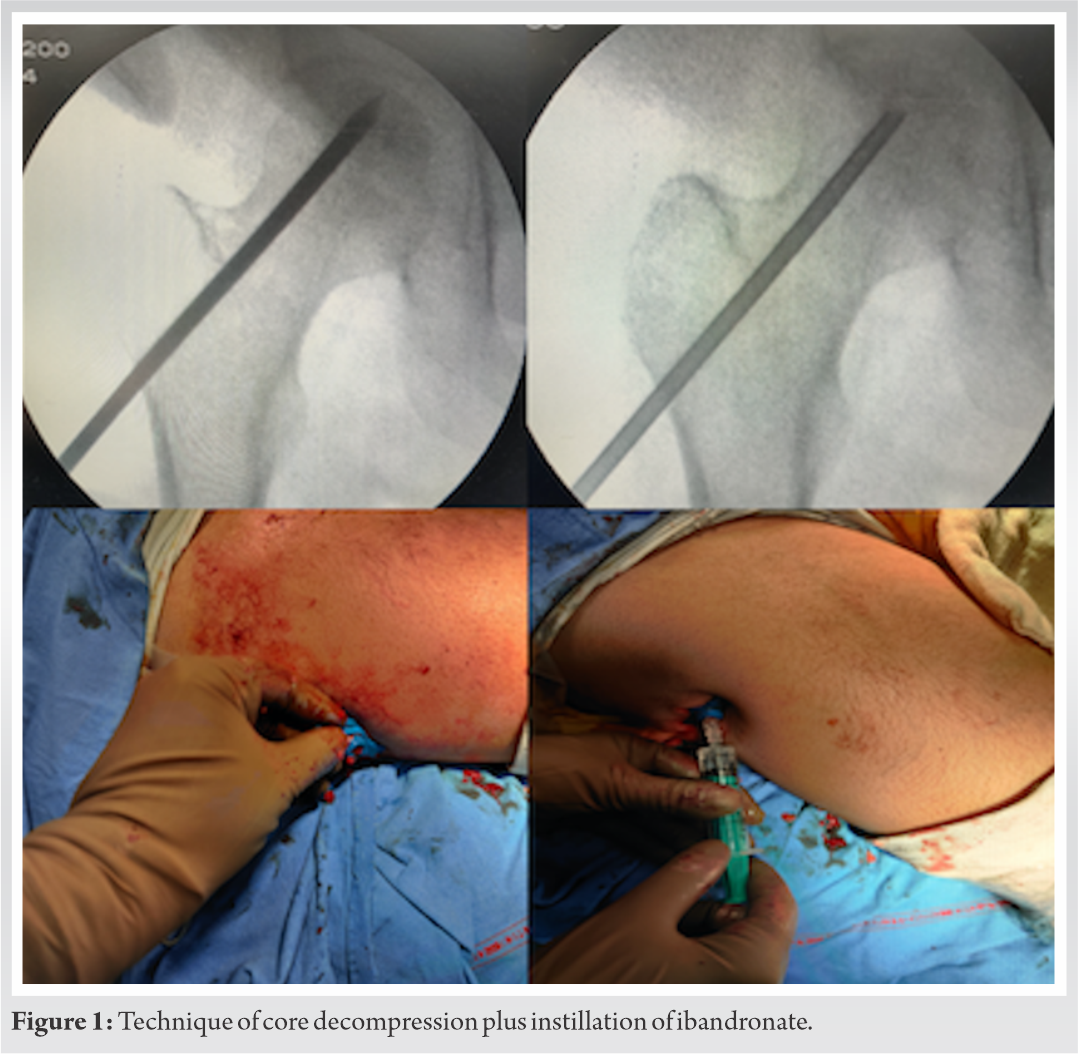
All the patients were operated under spinal anesthesia. In Bilateral cases, where arthroplasty was planned on one side, combined spinal-epidural anesthesia was used. The patients were operated in the supine position on a radiolucent table, with a radiolucent bag placed under the sacral area. Standard aseptic preparation was followed. Bilateral cases were done sequentially in the same sit-ting. A guide wire was placed over the skin anteriorly and the neck shaft angle was confirmed under C arm. A 2 cm incision was made at the base of the greater trochanter. Under C arm guidance, a 3.5 mm Steinman pin was used to drill 4–5 holes extending from the vastus lateralis ridge into the neck and up to the subchondral part of femoral head; the target was the necrotic area inside the head. Increased resistance felt while drilling followed by a sudden give, signified the proper placement of the pin, which was confirmed by anteroposterior and lateral views in the C arm. The tract created up to the main necrotic area was chosen for instillation of ibandronate. A Jamshidi needle (11 G) was inserted in that tract through which the drug was instilled. The dosage was based on animal data which suggest that 0.6 mg of ibandronate is sufficient [16]. We doubled the dose to adjust for wastage and instilled 1.2–1.5 mg of the drug. The ipsilateral side was raised to avoid back flow and bone wax was pushed into the tract after instillation followed by closure (Fig. 1).
Post-operative protocol
Postoperatively, the patients were not allowed to bear any weight on the operated side. In bilateral cases, mobilization was delayed till 6 weeks to allow the drill holes to heal. In unilateral cases, weight bearing on the non-operated side was allowed from post-operative day 2. If one side under-went arthroplasty, partial weight bearing was allowed on that side. The patient was encouraged to perform active ankle pumps, isometric quadriceps strengthening, and hip abduction/adduction exercises. Frequent side turns and pelvic lifting exercises were initiated to avoid bed sores in non-mobilized cases. Sutures were removed on day 14.
Follow-up
The patients included in this study were followed up after 2 weeks, 6 weeks, 6 months, and 1 year. The patients were evaluated clinically with the modified Harris hip score (MHHS) and visual ana-log scale (VAS); radiological evaluation was done with X-rays for assessing improvement or worsening of ONFH by one stage or subchondral collapse. DEXA scan was done for the hips to assess for any cortical signs of stress reactions/fractures due to ibandronate.
Statistical analysis
Due to the small sample size, we performed only two sets of comparisons. The pre-operative VAS score was compared to the score at last follow-up by means of a paired t-test. P<0.05 were consid-ered as significant. SPSS version 20 was used.
Results
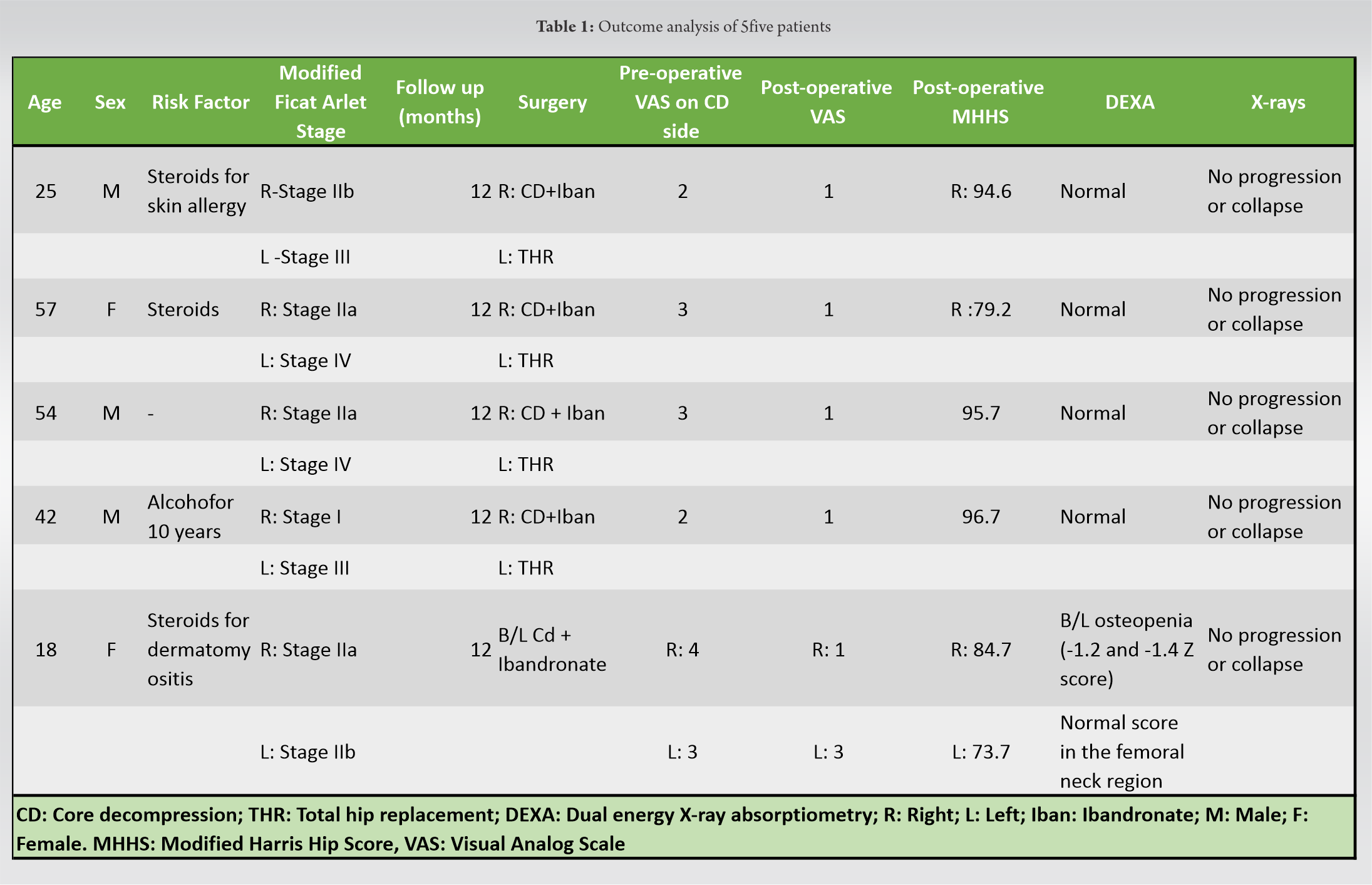
The present study included six hips in five patients (three females and 2 males) of ONFH, with mean of 39.2±17.28 (range from 18 to 57 years). Three patients had a history of steroid intake and one had consumed alcohol for 10 years; one patient was categorized as idiopathic. One hip be-longed to Stage I, three to Stage IIa, and two in the crescent phase (Stage II b) according to the modified Ficat and Arlet staging (Table 1). All the cases were bilateral ONFH, with total hip re-placements done for the contralateral hips in four cases. One case underwent bilateral CD + iband-ronate instillation.
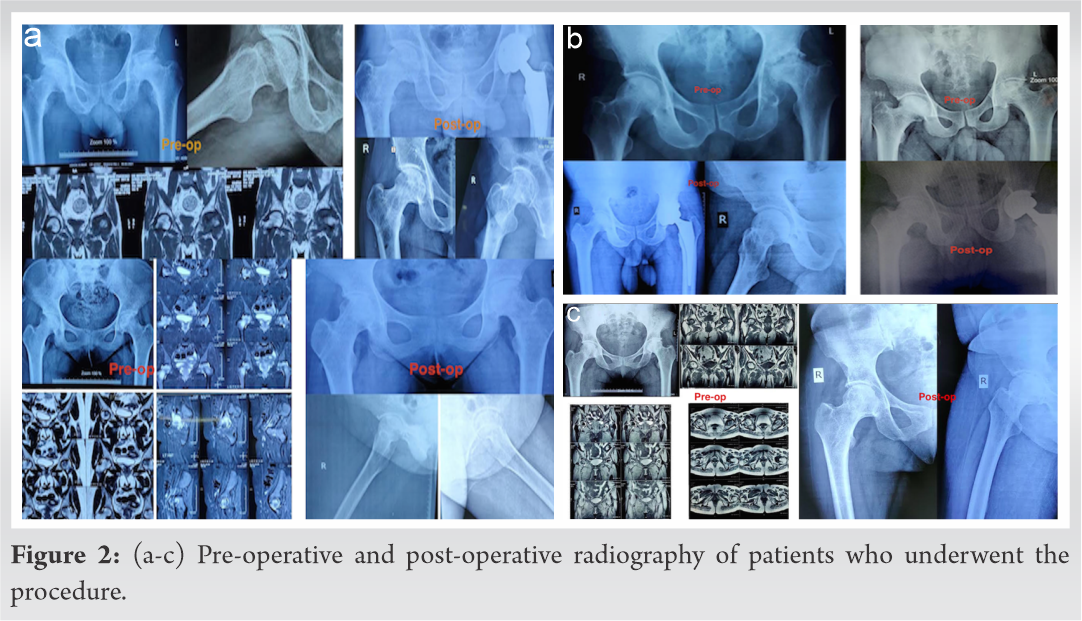
The radiographs of all the hips showed healing of the drill tracts, well-contoured femoral heads, and no signs of stage progression or collapse at last follow-up at 1 year (Fig. 2,3,4).
The mean pre-operative VAS score of the six hips which underwent CD was 2.8, which reduced to 1.3 postoperatively (p=0.00395). The average MHHS of the patients postoperatively was 87.4 (73.7–96.7).
The DEXA scan showed osteopenia in only one patient who underwent bilateral CD; however, the femoral neck Z scores were normal (−0.3 and −0.2 on the two sides, respectively). Probable reason could have been complete immobilization for 5 weeks in that particular patient. There were no indications of stress/atypical fractures such as cortical beaking/focal cortical changes laterally at the lesser trochanteric region in any patient.
There were no complications observed in any of the patients.
Avascular necrosis of the femoral head strikes an imbalance between bone formation and resorp-tion, which causes progression to collapse, distortion, and osteoarthritis [1]. The early stages tend to heal well with modern day adjuvants to CD such as BMAC and PRP; rates of worsening of AVN stage and conversion to arthroplasties tend to improve with these modalities [7, 8]. However, the high-associated costs continue to stress on the need for alternatives. The financial burden of arthroplasties itself is high and poorer patients specially in developing countries with lack of robust insurance mechanisms cannot afford THRs; this leads to further loss of work force due to associated functional limitations with negative impact on the economy [18].
Bisphosphonates have been shown to increase the trabecular bone volume by inhibiting bone re-sorption; there is improvement in the sphericity of the femoral head protecting its morphology [14]. Studies have shown that their usage may limit the proportion of patients who progress to femoral head collapse/distortion and need of THR [13, 14]. However, rare but severe complications include osteonecrosis of jaw and unusual atypical femoral fractures stress on the need for localized action of the drug to enable better bioavailability and lesser systemic toxicity. Ashraf et al. performed micro-CD of the necrotic area after safe surgical dislocation of affected hips and added intralesional zoledronic acid as treatment of AVN in 19 hips of 15 patients. They showed improved MHHS postoperatively with minimum follow-up of 2 years [19]. In Perthes disease which is ONFH of the femoral capital epiphysis, Sivakumar et al. explored local delivery of zoledronic acid in the epiphysis in two cases and found it effective in prevention of disease progression at 4 years of follow-up [20].
In the present series, we used ibandronate as it has been shown to maintain femoral head sphericity and adequate bioavailability in animal models of AVN hip. In addition, it has been shown by Fan et al that zoledronic acid can inhibit neovascularization, which is required for success of CD [21]. Hence, the combination of the two may not give optimal results.
Our short-term results were satisfactory with significant pain reduction in 5/6 hips and good to excellent functional outcomes along with adequate healing of the drill tracts. None of the cases had worsened clinico-radiologically and the patients were satisfied overall.
In terms of safety, one rare but worrying complication of localized delivery of ibandronate could be atypical fractures with delayed healing, with an incidence rate of 1.8-113 cases/100,000 [15]. These typically occur at the subtrochanteric region or the femoral shaft with transverse or short oblique fracture lines [22]. Bisphosphonates tend to increase tissue age by reducing turnover and diminish the overall toughness of the bone. There is increased mineral content at the lateral cortex which increases its brittleness; this increases chances of stress reaction and atypical fractures [22].
In systemic application these fractures occur on prolonged usage (>3 years); however, with targeted delivery, the incidence is unknown and no study has described the same. In our initial six hips, the DEXA scan did not show any pre-fracture hip lesions or focal lateral cortical changes in the form of periosteal changes or internal medullary changes. There were no prodromal symptoms such as ipsilateral thigh pain and serum calcium profiles were within normal limits.
Overall, our novel technique of combining intraosseous ibandronate with CD in pre-collapse stages of AVN has shown initial efficacy and safety. However, prolonged follow-up and increased number of included cases are needed to substantiate the method for recommending wider usage. Comparative trial with isolated CD is being done by the authors who will shed better light on additional benefit of this prospective adjuvant to CD.
The use of ibandronate as an adjuvant with CD has shown promising results. Although limited due to the small number of patients and short duration of follow-up, it appears to be safe and provides good clinical improvement and is a cheaper alternative. Further, large scale studies with higher number of patients are needed.
The use of ibandronate as an adjuvant with CD has shown promise as cheap alternative with safety and good clinical improvement.
References
- 1.Kumar P, Shetty VD, Dhillon MS. Efficacy of orthobiologic adjuvants to core decom-pression for hip preservation in avascular necrosis hip. J Hip Preserv Surg 2020;7:423-38. [Google Scholar]
- 2.Kumar P, Sen RK, Aggarwal S, Jindal K. Common hip conditions requiring primary to-tal hip arthroplasty and comparison of their post-operative functional outcomes. J Clin Orthop Trauma 2020;11:S192-5. [Google Scholar]
- 3.Fairbank AC, Bhatia D, Jinnah RH, Hungerford DS. Long-term results of core decom-pression for ischaemic necrosis of the femoral head. J Bone Joint Surg Br 1995;77:42-9. [Google Scholar]
- 4.Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res 2002;405:14-23. [Google Scholar]
- 5.Sugioka Y, Hotokebuchi T, Tsutsui H. Transtrochanteric anterior rotational osteotomy for idiopathic and steroid-induced necrosis of the femoral head. Indications and long-term results. Clin Orthop Relat Res 1992;277:111-20. [Google Scholar]
- 6.Yoon TR, Song EK, Rowe SM, Park CH. Failure after core decompression in osteone-crosis of the femoral head. Int Orthop 2001;24:316-8. [Google Scholar]
- 7.Aggarwal AK, Poornalingam K, Jain A, Prakash M. Combining platelet-rich plasma in-stillation with core decompression improves functional outcome and delays progression in early-stage avascular necrosis of femoral head: A 4.5-to 6-year prospective random-ized comparative study. J Arthroplasty 2021;36:54-61. [Google Scholar]
- 8.Jindal K, Aggarwal S, Kumar P, Rathod P. Core decompression with bone marrow aspi-rate concentrate in post collapse avascular necrosis of hip: A systematic review and meta-analysis. J Clin Orthop Trauma 2021;17:78-87. [Google Scholar]
- 9.Papavasiliou AV, Triantafyllopoulos I, Paxinos O, Tsoukas D, Kostantoulakis C. The role of cell therapies and hip arthroscopy in the management of osteonecrosis: An up-date. J Hip Preserv Surg 2018;5:202-8. [Google Scholar]
- 10.Smith SW, Meyer RA, Connor PM, Smith SE, Hanley EN Jr. Interobserver reliability and intraobserver reproducibility of the modified Ficat classification system of osteonecrosis of the femoral head. JBJS 1996;78:1702-6. [Google Scholar]
- 11.Epstein S, Zaidi M. Biological properties and mechanism of action of ibandronate: Ap-plication to the treatment of osteoporosis. Bone 2005;37:433-40. [Google Scholar]
- 12.Russell RG. Bisphosphonates: Mode of action and pharmacology. Pediatrics 2007;119:S150-62. [Google Scholar]
- 13.Agarwala S, Jain D, Joshi V, Sule A. Efficacy of alendronate, a bisphosphonate, in the treatment of AVN of the hip. A prospective open-label study. Rheumatology 2005;44:352-9. [Google Scholar]
- 14.Li D, Yang Z, Wei Z, Kang P. Efficacy of bisphosphonates in the treatment of femoral head osteonecrosis: A PRISMA-compliant meta-analysis of animal studies and clinical trials. Sci Rep 2018;8:1-11. [Google Scholar]
- 15.Khan AA, Kaiser S. Atypical femoral fracture. CMAJ 2017;189:E542-E. [Google Scholar]
- 16.Aya‐ya J, Athavale S, Morganpical femoral fracture. CMAJonates in the treatdistribu-tion, and effects of intraosseously administered ibandronate in the infarcted femoral head. J Bone Miner Res 2007;22:93-100. [Google Scholar]
- 17.Vandermeer JS, Kamiya N, Aya-ay J, Garces A, Browne R, Kim HK. Local administra-tion of ibandronate and bone morphogenetic protein-2 after ischemic osteonecrosis of the immature femoral head: A combined therapy that stimulates bone formation and de-creases femoral head deformity. JBJS 2011;93:905-13. [Google Scholar]
- 18.Ghosh S, Aggarwal S, Kumar V, Patel S, Kumar P. Epidemiology of pelvic fractures in adults: Our experience at a tertiary hospital. Chin J Traumatol 2019;22:138-41. [Google Scholar]
- 19.Ashraf M, George J, Sha II. Micro-core decompression combined with intralesional zoledronic acid as a treatment of osteonecrosis of femoral head: A novel technique. J Orthop Traumatol Rehabil 2021;13:21. [Google Scholar]
- 20.Sivakumar R, Somashekar V, Singhi PK, Chidambaram M. Local delivery of single dose intra epiphyseal bisphosphonates to prevent the progression of legg-calve-perthes disease-case series. J Orthop Case Rep 2020;10:65. [Google Scholar]
- 21.Fan M, Jiang W, Wang A, Wang Y, Peng J, Zhang L, et al. Effect and mechanism of zoledronate on prevention of collapse in osteonecrosis of the femoral head. Zhongguo yi xue ke xue Yuan xue bao 2012;34:330-6. [Google Scholar]
- 22.Larsen MS, Schmal H. The enigma of atypical femoral fractures: A summary of current knowledge. EFORT Open Rev 2018;3:494-500. [Google Scholar]