Hidradenocarcinomas are very rare and aggressive tumors which must be correctly diagnosed and treated, so we must consider that if the treatment of its benign precursors is not radical enough, malignant transformation and aggressive recurrences such as hidradenocarcinomas can occur, making their management much more complex and worsening prognosis.
Dr. Jaime Sanchez del Saz, Calle Profesor Martin Lagos, s/n, Madrid - 28040, Spain. E-mail: jsdelsaz@gmail.com
Introduction: Hidradenocarcinomas are very rare and aggressive soft-tissue tumors, originated from sweat gland cells, which are located most frequently in head and neck, being their appearance at the extremities rare. This kind of tumor usually appears de novo and very few cases have been reported until now of appearance over benign lesions such as hidradenomas. Malignancy progression rate of hidradenomas is unknown, and this benign lesion has clinical and histopathological characteristics in common with hidradenocarcinomas that could lead to misdiagnosis. Hidradenocarcinomas have a very poor survival rate; therefore, an early diagnosis is essential for a better prognosis, and that is the reason why hidradenomas should be widely excised from the beginning, instead of performing marginal resections of this lesions that could lead to an aggressive recurrence.
Case Presentation: Here is a case report of a 27-year-old woman diagnosed with a hidradenocarcinoma over a previously excised hidradenoma in the right foot. The diagnosis was made after right pelvic and inguinal lymphadenopathies appeared few months after a new small asymptomatic lump appeared at the same place in the sole of the right foot were the excised hidradenoma five years before was located. Lymph node biopsy was performed, with histopathological diagnosis of hidradenocarcinoma metastasis. Surgical local wide excision of the lump at the foot and lymphadenectomies was performed. Histopathological analysis of the samples confirmed the diagnosis of hidradenocarcinoma. The patient later received adjuvant radiotherapy and after one year there are no signs of disease recurrence.
Conclusion: Many questions remain uncertain about the management and treatment of hidradenocarcinomas due to the rarity of this type of tumor. Although targeted molecular therapies have shown promising results, more studies in this field are necessary. An early diagnosis and differentiation from its benign counterparts that allow local control of the disease before spreading is essential to improve survival rates.
Keywords: Hidradenocarcinoma, hidradenoma, foot, orthopaedic oncology.
Hidradenocarcinomas are aggressive soft-tissue malignant tumors derived from eccrine or apocrine cells of the sweat glands. They are extremely rare within a group of already rare tumors such as sweat gland tumors. Hidradenocarcinomas stand for only 6% of eccrine sweat gland tumors, less than 0.001% of all tumors, and less than 0.01% of all skin tumors [1].
The most frequent age of onset is between 55 and 70 years, with a slight female gender predominance. There are no differences based on race [2, 3]. The most common location for this type of tumor is the head and neck, being its appearance in the extremities rare. It has been also described at the trunk, abdomen, or elbow [2]. Genetic mutations clearly linked to this tumor have not been found, although some of them related to its appearance are mutations in MALM2, p53, T53, and the 11;19 translocation (CRTC-1). No exogenous risk factors have been clearly linked to this tumor, although radiation could be one of them [3].
This type of tumor presents a first indolent, non-specific, clinical phase, being a usually superficial tumor (intradermal), with a size between 1 and 5 cm, well defined, and slow growing over a variable period (from months to decades) during which patients are usually asymptomatic or present mild discomfort. This first phase is followed by a second symptomatic phase with aggressive behavior of the tumor, which spreads regionally (with pain, bleeding, and even ulceration of the lesion) and at distance (mainly to lymph nodes), worsening its prognosis, with a survival rate of 30% at 5 years after surgery [2, 3, 4, 5]. Histologically, it presents a combination of spindle cells with eosinophilic cytoplasm and large clear cells that show atypical mitotic figures and nuclear pleomorphism, which can express epithelial membrane antigen, carcinoembryonic antigen, and S100 protein. Ductal structures or cystic spaces are not usually seen, and malignancy data such as vascular and perineural invasion, deep extension, or necrosis can be seen, but areas of nodular hidradenoma can also be seen, which can lead to misdiagnoses [5, 6]. Typically, this type of tumor appears de novo, being very rare its origin over a previous benign lesion, although three other cases over a preexisting hidradenoma have been already described to the best of our knowledge, as in our case [5, 6, 7]. However, none of them had previously received surgical treatment, being another singularity of our case the fact that the patient had already undergone surgery years ago for the treatment of this benign lesion.
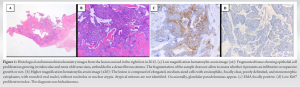
We present the case of a 27-year-old woman with personal history of marginal surgical excision of a tumor in the sole of her right foot in 2015, which received the histopathological diagnosis of hidradenoma (Fig. 1). The patient did not refer other personal history of interest.
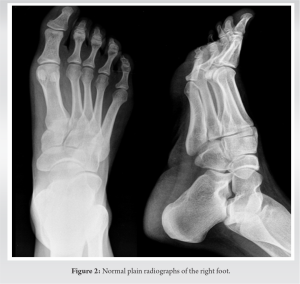
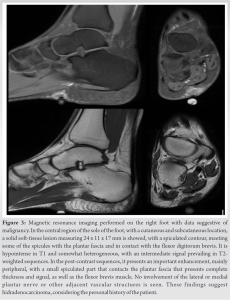
She had remained asymptomatic until August of 2019 when she noticed the appearance of an approximately 1 cm and non-mobile lump in the same place where the surgical scar was located at her right foot. It presented slow growth for months, causing her discomfort when walking. The only sign appreciated was the effusion of little amount of serous fluid through the lump. Finally, the reason why she consulted again at the beginning of the year 2020 was the recent appearance and increasing size of a painless lymphadenopathy in the right inguinal region. First, we obtained plain radiographies of the right foot without finding signs of bone involvement (Fig. 2). Afterwards, a magnetic resonance imaging was performed showing a new subcutaneous lesion in the right foot sole (Fig. 3).
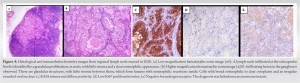
Given the patient’s personal history, it was decided to perform an open biopsy of the inguinal lymphadenopathy, obtaining the histopathological diagnosis of hidradenocarcinoma metastases (Fig. 4).
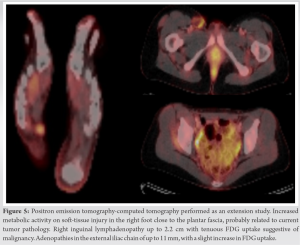
For this reason, extension study of the disease was carried out with a positron emission tomography-computed tomography (PET-CT), which confirmed the suspicion of malignant tumoral injury at the right foot and distant dissemination of the tumor at the right iliac and inguinal lymph nodes, without any another affected location (Fig. 5).
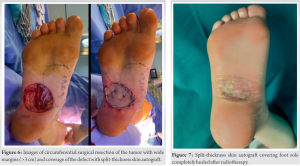
Subsequently, the patient underwent surgery in collaboration with surgeons from the general surgery and plastic surgery departments. In the first stage, a laparoscopic right pelvic lymphadenectomy was performed followed by an open right inguinal lymphadenectomy. In the second stage, a wide circumferential excision of the tumor was performed at the sole of the right foot, including part of the plantar fascia, but without excising the flexor muscle compartment of the foot and fingers. Finally, the defect was covered with a split-thickness skin graft obtained from the right thigh of the patient. Since the defect was not located in a weight bearing surface, free flap or local flap reconstruction was not chosen (Fig. 6).
The histopathological analysis of the surgical specimen revealed a neoplastic proliferation with variable cellularity (combination of squamous cells, spindle cells, and others with eosinophilic cytoplasm) and infiltrative growth pattern forming solid nests and cords of cells of variable size and morphology. Glandular structures with eosinophilic secretion were also found. Mitotic activity was low and there was neoplastic lymphovascular invasion but no perineural infiltration. Surgical margins were free of neoplastic involvement. All these findings were consistent with hidradenocarcinoma diagnosis. In the immunohistochemical study, there was no expression of estrogen, androgen nor human epidermal growth factor receptor 2 (HER-2) receptors. The lymph nodes samples revealed the presence of metastases in five out of six iliac and in two out of eleven inguinal lymph nodes. After the intervention, non-weight bearing of the intervened limb was indicated for three weeks to ensure skin graft integration, and once the graft was secured in the sole of the foot, adjuvant radiotherapy was started. The defect was properly covered, and the patient started a normal life and daily standing work two months after completing radiotherapy treatment (Fig. 7). Because of the lymphadenectomy, the patient developed lymphedema of the lower right limb that required treatment with and specific compression stocking. No other post-operative physiotherapy treatment was necessary to retrieve patient’s habitual daily activity. Close clinical controls and imaging tests with PET-CT were carried out during the first year postoperatively and recurrence-free survival has been achieved for at least one year with this treatment protocol.
Hidradenocarcinoma is an aggressive malignant tumor derived from sweat gland cells, which is why it has also been called eccrine clear cell carcinoma, malignant nodular hidradenoma, malignant clear cell hidradenoma, malignant clear cell acrospiroma, or primary mucoepidermoid cutaneous carcinoma [2].
The key in the management of this type of tumor is early diagnosis and treatment. However, the paucity of symptoms it produces in its first phases makes initial consult long-lasting. This fact, together with the presence of clinical and histological characteristics in common with other types of lesions, can hinder the diagnostic process, especially in its initial stages. Consequently, it is often diagnosed once it has spread, associating a worse prognosis. The differential diagnosis includes a wide range of tumors such as lipomas, hemangiomas, lymphangiomas, squamous cell carcinomas, basal cell carcinomas, malignant melanomas, various malignant adnexal carcinomas, and other visceral tumors with skin metastases [1].
The malignancy rate of hidradenomas is unknown and the literature describes cases with aggressive behavior other than the benign characteristics that it usually has, which is why extensive excision of hidradenomas is currently recommended, especially in those cases of recurrent lesions [5].
From the immunohistochemistry point of view, this type of tumor can express androgen (36%), estrogen (27%), progesterone (16%) receptors, epidermal growth factor receptor or EGFR (85%), and the HER-2 receptor (12%). These receptors are important for the application of targeted therapies.
The gold standard of treatment is still surgical excision with a tumor-free margin of at least 3 cm. However, approximately 66% of patients present distant extension of the tumor (39% to lymph nodes and 28% to other distant organs that include bone, lung, pleura, skin, and among others), and the local recurrence rate of this type of tumor is high (up to 50%). Therefore, surgical treatment of the tumor is currently combined with regional lymphadenectomy whenever possible if there is regional lymph node involvement, and even prophylactically [2, 3, 4, 8, 9, 10].
Sentinel node biopsy can be useful in the early diagnosis of lymph node metastases in the subclinical phase in those cases with lesions with data suggesting a high risk of malignancy, such as invasion more than 2 mm in depth, perineural invasion, and histological dedifferentiation [2, 8, 9].
Adjuvant radiotherapy is usually reserved for those cases in which surgery (for both the tumor and the affected lymph nodes) is not recommended due to anatomical or functional reasons, in cases with affected surgical margins, perineural, vascular or lymphatic invasion, deep infiltration, highly anaplastic morphology, and in tumor recurrences. High doses (50-70 Gy) are used, which can cause local complications, including soft tissue necrosis, osteoradionecrosis, and secondary neoplasms. The results of radiotherapy in this type of tumors are contradictory, so its therapeutic implication continues to be debated [3, 10, 11, 12]. The most widely used chemotherapy regimens include 5-fluorouracil and capecitabine. To date, the effectiveness of chemotherapy alone or in combination with radiotherapy remains doubtful in terms of disease control and survival rates [3, 10, 12, 13], although Lerner et al describe a case of unresectable hidradenocarcinoma that presented a sustained complete remission after 3 months of capecitabin therapy [14].
Other systemic therapies have been described, such as targeted therapy with trastuzumab (a monoclonal antibody directed against the HER-2 receptor), which appears to be one of the most promising, as it appears to contribute to the stabilization of disease progression [2].
Hidradenocarcinoma is an extraordinarily rare tumor, so many questions related to its diagnosis and treatment remain unresolved today. Its aggressive behavior, with frequent metastases and local recurrences, makes it essential to carry out an early and precise diagnosis of the tumor, differentiating it from its benign counterparts, which can lead to its wide surgical resection. This allows the lesion to be surgically treated before its distant spread occurs to obtain a better prognosis. However, there is no standardized therapeutic protocol given the rarity of this tumor. Targeted molecular therapies appear to be the future of the treatment for this type of tumor, given the uncertain effectiveness of chemotherapy and radiotherapy, having yielded promising results for the control of the spread of this rare tumor.
Hidradenocarcinomas are very rare and aggressive tumors with poor survival rates. Their most frequent origin is de novo but, in some cases, it can be originated over benign lesions. These lesions must be correctly diagnosed and treated. If treatment of its benign precursors is not radical enough, malignant transformation and aggressive recurrences such as hidradenocarcinomas can occur, making management of this lesions much more complex and worsening prognosis.
References
- 1.Hernández-Pérez E, Cestoni-Parducci R. Nodular hidradenoma and hidradenocarcinoma. A 10-year review. J Am Acad Dermatol 1985;12:15-20. [Google Scholar]
- 2.Gauerke S, Driscoll JJ. Hydradenocarcinomas: A brief review and future directions. Arch Pathol Lab Med 2010;134:781-5. [Google Scholar]
- 3.Soni A, Bansal N, Kaushal V, Chauhan AK. Current management approach to hidradenocarcinoma: A comprehensive review of the literature. Ecancermedicalscience 2015;9:517. [Google Scholar]
- 4.Płachta I, Kleibert M, Czarnecka AM, Spałek M, Szumera-Ciećkiewicz A, Rutkowski P. Current diagnosis and treatment options for cutaneous adnexal neoplasms with apocrine and eccrine differentiation. Int J Mol Sci 2021;22:5077. [Google Scholar]
- 5.Lim SC, Lee MJ, Lee MS, Kee KH, Suh CH. Giant hidradenocarcinoma: A report of malignant transformation from nodular hidradenoma. Pathol Int 1998;48:818-23. [Google Scholar]
- 6.Oviedo Ramírez I, Ferri-Ñíguez B, Martínez-Barba E. Hidradenocarcinoma originating from nodular hydradenoma: Description of a case. Rev Esp Patol 2010;43:47-51. [Google Scholar]
- 7.Sami G, Baline K, Hali F, Diouri M, Marnissi F, Chiheb S. Hidradénocarcinome develops in a pre-existing hydradenome. Ann Dermatol Venereol 2019;146:A171. [Google Scholar]
- 8.Kane B, Adler E, Bhandari T, Rose M, DiGuglielmo N, Sun X. Malignant hidradenocarcinoma in the lower extremity: A case report of a rare tumor. J Foot Ankle Surg 2018;57:618-21. [Google Scholar]
- 9.Rafols M, Mejia O, Oh KS, Bendixen B, Jorge I, Narayanan S. An unusual case of lower extremity clear cell hidradenocarcinoma. Case Rep Surg 2020;2020:6192109. [Google Scholar]
- 10.Mir Khan B, Mansha MA, Ali N, Abbasi AN, Ahmed SM, Qureshi BM. Hidradenocarcinoma: Five years of local and systemic control of a rare sweat gland neoplasm with nodal metastasis. Cureus 2018;10:e2884. [Google Scholar]
- 11.Lalya I, Hadadi K, Tazi EM, Bazine A, Andaloussy K, Elmarjany M, et al. Radiotherapy on hidradenocarcinoma. N Am J Med Sci 2011;3:43-5. [Google Scholar]
- 12.Miller DH, Peterson JL, Buskirk SJ, Vallow LA, Ta R, Joseph R, et al. Management of metastatic apocrine hidradenocarcinoma with chemotherapy and radiation. Rare Tumors 2015;7:6082. [Google Scholar]
- 13.Amel T, Olfa G, Faten H, Makrem H, Slim BA, Moncef M. Metastatic hydradenocarcinoma: Surgery and chemotherapy. N Am J Med Sci 2009;1:372-4. [Google Scholar]
- 14.Lerner A, Beckford A, Ugent S, Goldberg L, Jalisi S, Demierre MF. Complete response of metastatic malignant hidradenocarcinoma to capecitabine treatment. Arch Dermatol 2011;147:998-9. [Google Scholar]