We can learn the aim of rehabilitation in oncologic spinal cord injury patient to improve personal quality of life.
Dr. Gabriella Fizzotti, Spinal Unit, ICS Maugeri SPA SB, Institute of Pavia, Istituto di Ricovero e Cura a Carattere Scientifico, Pavia, Italy, Europe. E-mail: gabriella.fizzotti@icsmaugeri.it
Introduction: Neoplastic patients with spinal cord injury (SCI) who commonly present at rehabilitation units exhibit different characteristics from traumatic SCI patients but the rehabilitation results are similar. The aim of this paper is to describe the rehabilitation outcome in a patient with paraplegia caused by giant cell tumor of bone (GCTB) located at D11 level of spine.
Case Report: The patient was a 26-year-old Chinese man who had a history of back pain complicated by paraplegia. Magnetic resonance imaging (NMR) evidenced giant cell tumor removed surgically. Individual rehabilitation program aimed to recovery walking autonomy was proposed to the patient.
Conclusion: A case report recovered a good grade of autonomy in walking function and returned to daily activities.
Keywords: Giant cell tumor, rehabilitation, spinal cord injury.
Giant cell tumor of bone (GCTB) is locally aggressive primary tumor that is associated with variable aggressiveness [1]. This tumor is approximately 5–6% of primary bone tumors [2]. It occurs most frequently in young adults, between 20 and 40 years old and in the female sex [3]. GCT is most often found in the epiphysis of the long bones; the most common locations are the distal femur, the proximal tibia, the distal radius, and the sacrum [4]. The incidence of the spine involvement in the vertebrae above the sacrum is quite low, from 1% to 2.9%, equal incidence is seen in all three spinal segments (cervical, dorsal, and lumbar) [5, 6]. GCTB is radiologically osteolytic lesions, the radiological diagnosis of GCT is done with radiography and magnetic resonance imaging (MRI) with contrast media (MRI). The main radiographic differential diagnoses include brown tumor of hyperparathyroidism, metastatic disease, hematologic malignancies, and aneurysmal bone cyst [7]. Histological analysis of a biopsy tissue sample is necessary to obtain diagnosis, tumor classification, and grading. The treatment of choice is total resection with appropriate reconstruction or curettage, in cases of subtotal resection radiation therapy can be performed; it will depend on level of involvement. If the tumor is incompletely removed, there is an increased risk of local recurrence and metastasis, approximately 1%–6% tumors metastasize, most commonly to lung [2]. In patients with giant cell tumor of the spine, the most common symptom is back pain, other symptoms may be neurological deficit such as paraplegia, bowel and bladder dysfunction, and other neurologic symptoms due to spinal cord compression or structural deformity of the spine. Spinal cord injury (SCI) can be of traumatic or non-traumatic origin, classified as complete or incomplete. The leading causes of non-traumatic SCI are neoplastic tumors and degenerative conditions of the spinal column, followed by vascular and autoimmune disorders. The neoplastic compression of the spinal cord in 85% is due to metastatic lesions, while the remaining 15% to primary neoplastic lesions of the spine [8]. Neoplasm of the spinal cord growing inside and compressing the spinal canal [9], this damage can cause a combination of the upper motor neuron weakness, spasticity, sensor loss, and sphincter disturbance below the level of the lesion.
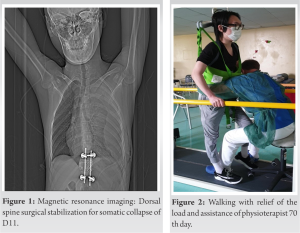
In this study, we have evaluated a patient admitted to the spinal unit of the clinical scientific institute Maugeri of Pavia in the period between April and October 2021. The patient was a 26-year-old Chinese man, who had a history of sudden back pain at dorsal level immediately complicated by paraplegia. He had no medical history before this event. MRI of the dorsal spine showed somatic collapse of D11 with retraction of the posterior wall and compression of the medullary cord (Fig. 1). On February 14, 2021, patient was operated with decompressive D10-D12 laminectomy and lateral mass screw-rod arthrodesis. Post-surgical course was characterized both no motor functional recovery of the lower limbs and normal sensation to light touch, temperature, and pain. The histological examination of biopsies resulted positive for GCT of bone. A case report was performed with D11 tumorectomy in lateral access with positioning the inter-body mesh and he was transferred on April 6, 2021, to U.O. Spinal for starting the rehabilitation program.
SCIs were classified as complete and incomplete by the American spinal injury association (ASIA) classification [10]. The ASIA scale grades patients based on their functional impairment from A to E, where A represents the greatest impairment and E represents the normal condition. Physical examination revealed paraplegia and atrophy of the lower limb muscles. He demonstrated hyperreflexia in the lower extremities and spasticity of muscles and joints in both limb. Sensation to light touch, temperature, and pain were normal. Bladder function was impaired: Patient was unable to empty his bladder completely than a permanent indwelling bladder catheter was placed. A case report was classified in ASIA B. NMR evidenced focal signal alteration of the spinal cord at D11 level, extending in the craniocaudal direction for about 7 mm hyperintense in the long TR sequences and not equipped with contrast impregnation. Needle electromyography of the right and left external anal sphincter muscle, innervated by S3, S4, S5 roots, evidenced chronic denervation signs, without denervation activity at rest superficial anal reflex was present. Motor evoked potentials, of the lower limbs showed a pathology of the first motor neuron and/or intra-rachide of the second motor neuron. Somatosensory evoked potentials, (SEPs) resulting from stimulation of the dorsal nerve of the penis, evidenced the absence of cortical response (P1). Peripheral conduction (ankle-popliteal fossa) and cortical response (P40) resulting from electrical stimulation of the tibial nerve at the ankle resulted absent. Individual rehabilitation program (PRI) aimed to: Improve motor performances and the grade of autonomy, to increase the control of pain and spasticity. Patient was educated to stretching the lower limbs and training postural passages verticalization is an important factor of early rehabilitation in the patients with SCI before to begin walking training.
The time walking test (TWT) evaluated the improvement in walking endurance. The TWT was developed by the American Thoracic Society and it was officially introduced in 2002, coming along with a comprehensive guideline [11]. The TWT is a sub-maximal exercise test used to assess aerobic capacity and endurance. A case report evidenced an improvement in walking performance during PRI. (Table 1) The walking index for spinal cord injury (WISCII) is a scale that measures the type and amount of assistance (in terms of requirements of assistive devices, or human helpers) required by a person with SCI for walking [12]. It is an ordinal scale which rates people with SCI from being unable to walk to independent walking and designed to indicate the grades of impairment occurring after SCI and their relationship to the function of walking [13]. The results in TWT and WISCII evidenced an improvement in independent walking (Table 1). At the end of rehabilitation program, SEPs have been controlled. P40 resulting present after stimulation of the tibial nerve at the ankle evidenced with long latency.
We presented this case report for two reasons: The atypical site of GCTB and the satisfactory result of rehabilitation program. Physiotherapy treatment included: Exercises to strengthen weak muscles in the legs and trunk. Muscle stretching to reduce pain and stiffness and to prevent soft-tissue contractures was frequently adopted in the starting phase of rehabilitation. During the physical activity, the patient wore a C35 type orthopedic corset to transfer himself in his wheelchair, to keep exercising do not stop training Gait retraining with the use of mobility aids such as crutches or walking frame was proposed after 45 days of rehabilitation. Physiotherapist included walking training with the use of a walker with antebrachial support: Taurus type. A case report performed the 1st TWT after 90 days of rehabilitation program. Physiotherapy treatment included training in climbing and descending stair, patient was able to walk with the use of walker after a period of 4 months. The C35 corset was no longer necessary because pain improved. After a period of 140 days, patient set up walking with two Canadian style canes. A case report reached the rehabilitation outcome of walking independently in a period of 6 months. Neoplastic SCI patients who commonly presented at rehabilitation units exhibit different characteristics from traumatic SCI patients but the rehabilitation results are similar. The cord lesion was at D11/12 but the muscles affected are innervated by nerve roots L2–S1. The spinal cord enlargement that corresponds to the legs, the lumbosacral enlargement, resides in the vertebral column from approximately D10 to the conus at L1/2. A lesion of the thoracic cord below T10, such as the T11/12 lesions described, can therefore, in theory, damage anterior horn cells innervating the L2–S2 myotomes [14]. The anterior horn cells lie in the terminal territory of segmental sulcal arteries (branches of the anterior spinal artery) from which they receive their blood supply [15]. Anterior horn cells are thus more vulnerable to circulatory insufficiency than other cell types that reside within the spinal cord at the same level. McKinley WO, Huang ME, and Brunsvold KX showed that the assessment and treatment of patients with neoplastic versus traumatic SCI appeared to have taken a similar length of stay [16]. According to Tay SS and colleagues, cancer patients benefit as much as non-cancer patients in undergoing a rehabilitation program. More patients should be admitted to such program and these program should be better structured and refined [17]. Furthermore, Marciniak and colleagues evidenced that patients with cancer can result in significant functional gains [18]. In the study of Afsar SI, Cosar SNS, Yemisci OU, and Boluk H documented that Incomplete SCI was significantly higher in the neoplastic group when compared with the traumatic group [19]. A case report was 26-year-old male, in opposition to the female prevalence in GCTB epidemiology. Denosumab is the new introduced drug for the treatment of GCTB. A case report was carefully monitored for the possible onset of secondaries. We decided to start the oncological drug treatment only in GCTB locations that can be difficult to attack surgically. Although there are many studies of SCI resulting from neoplastic spinal cord compression, the definition of different rehabilitative outcome is still difficult. The sequence of different and consequential outcome of PRI of case report evidenced the utility of PRI in oncological SCI (Figs. 2-5). Our case report describes the association between SCI and rehabilitation and suggests to apply the PRI to patients with GCTB.
The patient has achieved the outcome of PRI in relation to the level of the lesion and personal motivation. He is able to stand up and walk for medium-long distances using a walker or alternatively two Canadian sticks. He can climb two or three floors of stairs with minimal help and manages his body care with adequate times. A case report is not representative, but point to a relationship that is worth exploring on a larger research group. SCI in GCTB may present a rehabilitative recovery following a PRI.
The atypical site of GCTB and the satisfactory result of rehabilitation program make the case report interesting. Patients with SCI secondary to cancer patients benefit as much as non-cancer patients in undergoing a rehabilitation program.
References
- 1.Mavrogenis AF, Igoumenou VG, Megaloikonomos PD, Panagopoulos GN, Papagelopoulos PJ, Soucacos PN. Giant cell tumor of bone revisited. SICOT J 2017;3:54. [Google Scholar]
- 2.Palmerini E, Picci P, Reichardt P, Downey G. Malignancy in giant cell tumor of bone: A review of the literature. Technol Cancer Res Treat 2019;18:1533033819840000. [Google Scholar]
- 3.Fletcher CD, Unni K, Mertens F editors. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon, France: IARC Press; 2002. [Google Scholar]
- 4.Sobti A, Agrawal P, Agarwala S, Agarwal M. Giant cell tumor of bone-an overview. Arch Bone Jt Surg 2016;4:2-9. [Google Scholar]
- 5.Bhojraj SY, Nene A, Mohite S, Varma R. Giant cell tumor of the spine: A review of 9 surgical interventions in 6 cases. Indian J Orthop 2007;41:146-50. [Google Scholar]
- 6.Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am 1987;69:106-14. [Google Scholar]
- 7.Greenspan A, Remagen R. Differential Diagnosis of Tumors and Tumor-like Lesions of Bones and Joints. Philadelphia, (PA): Lippincott-Raven; 1998. [Google Scholar]
- 8.World Health Organization. International Spinal Cord Society. International Perspectives on Spinal Cord Injury. Geneva: World Health Organization; 2013. [Google Scholar]
- 9.Öztop F. Omurga tümörlerinin patolojisi. In: Zileli M, Özer AF, editors. Omurilik ve Omurga Cerrahisi. Cilt. 2. İzmir: Meta Basım Matbaacılık Hizmetleri; 2002. p. 957-74.10. [Google Scholar]
- 10.Kirshblum SC, Waring W, Biering-Sorensen F, Burns SP, Johansen M, Schmidt-Read M, et al. Reference for the 2011 revision of the international standards for neurological classification of spinal cord injury. J Spinal Cord Med 2011;34:547-54. [Google Scholar]
- 11.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111-7. [Google Scholar]
- 12.Ditunno JF Jr., Ditunno PL, Graziani V, Scivoletto G, Bernardi M, Castellano V, et al. Walking index for spinal cord injury (WISCI): An international multicenter validity and reliability study. Spinal Cord 2000;38:234-43. [Google Scholar]
- 13.Ditunno PL, Scivoletto G, Patrick M, Dijkers M, Barbeau H, Burns AS, et al. The walking index for spinal cord injury (WISCI/WISCI II): Nature, metric properties, use and misuse. Spinal Cord 2013;51:346-55. [Google Scholar]
- 14.Kok CY, Chandrashekar H, Turner C, Manji H, Rossor AM. Can compressive thoracic cord lesions cause a pure lower motor neurone syndrome? Pract Neurol 2019;19:72-4. [Google Scholar]
- 15.Martirosyan NL, Feuerstein JS, Theodore N, Cavalcanti DD, Spetzler RF, Preul MC. Blood supply and vascular reactivity of the spinal cord under normal and pathological conditions. J Neurosurg Spine 2011;15:238-51. [Google Scholar]
- 16.McKinley WO, Huang ME, Brunsvold KT. Neoplastic versus traumatic spinal cord injury: An outcome comparison after inpatient rehabilitation. Arch Phys Med Rehabil 1999;80:1253-7. [Google Scholar]
- 17.Tay SS, Ng YS, Lim PA. Functional outcomes of cancer patients in an inpatient rehabilitation setting. Ann Acad Med Singap 2009;38:197-201. [Google Scholar]
- 18.Marciniak CM, Sliwa JA, Spill G, Heinemann AW, Semik PE. Functional outcome following rehabilitation of the cancer patient. Arch Phys Med Rehabil 1996;77:54-7. [Google Scholar]
- 19.Afsar SI, Cosar SN, Yemişçi OU, Bölük H. Inpatient rehabilitation outcomes in neoplastic spinal cord compression vs. traumatic spinal cord injury. J Spinal Cord Med 2020;45:221-9. [Google Scholar]