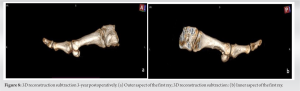
Full resection of a tenosynovial giant cell tumor may lead to adequate bone remodeling of a bony erosion, caused by mechanical pressure, without need of further intervention.
Dr. Maria Tsatlidou, Department of Orthopaedic, St Luke’s Hospital, Panorama, Thessaloniki, Greece, Thessaloniki, Greece. E-mail: maria.tsatlidou@yahoo.com
Introduction: The term tenosynovial giant cell tumor encompasses a group of rare soft-tissue tumors. A new classification divides the group in localized and diffuse type, depending on the involvement of the surrounding tissues. Due to the unclear origin and heterogeneity in extend of the diffuse-type giant cell tumors, there is only limited evidence on the tumor-specific treatment. Thus, every case report has an added value toward setting disease-specific guidelines.
Case Report: Presentation of a diffuse type tenosynovial giant cell tumor encircling the first metatarsal. The tumor had mechanically eroded the plantar aspect of the distal metaphysis, with no signs of tumor spread. After an open biopsy, resection of the mass was performed without debriding or resecting the first metatarsal. Repeat imaging postoperatively showed no recurrence at 4-year follow-up and a bony remodeling of the lesion.
Conclusion: Bone remodeling is possible after complete resection of diffuse tenosynovial giant cell tumor when the erosion is caused by mechanical pressure and no intraosseous expansion of the tumor is present.
Keywords: Diffuse-type tenosynovial giant cell tumor, first metatarsal, bony erosion, bone remodeling.
The term tenosynovial giant-cell tumor (TGCT) encompasses a group of soft-tissue tumors that can arise from the synovial lining of the joint, tendon, or bursa. According to the updated 2013 World Health Organization system for the classification of soft-tissue tumors, these can present as either localized-type or diffuse-type, depending on the extent of synovial involvement. Furthermore, both forms can arise intra-articularly or extra-articularly. In diffuse tumors however this origin may not always be clear [1, 2]. The incidence of dtGST is 4–8.4 per million person-years. Despite their rarity, they represent the most common soft-tissue tumors of the foot and ankle [3, 4]. Historically, a considerable heterogeneity of the relevant terminology has hampered the accumulation of large numbers of cases in patient cohorts and multicenter studies. As a result, in the literature, there is an abundance of case reports with few only large case series. Therefore, decision-making in individual cases follows general oncological principles rather than disease-specific guidelines based on strict recommendations. We present a case of a diffuse type giant cell tumor around the first metatarsal with a bone erosion at the level of the distal metaphysis. Following resection of the tumor without ray amputation, there was evidence of bony remodeling of the metatarsal.
A 44-year-old previously healthy female presented reporting a slowly growing mass at the dorsum of her right midfoot (Fig. 1).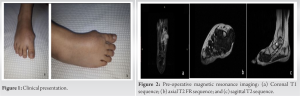
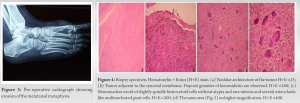
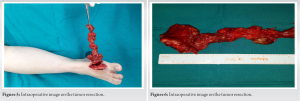

A bony GST affecting the first metatarsal is quite rare. In a series of 18 patients with GCT of the foot and ankle, the authors report no case of involvement of the first metatarsal [5]. In our case, a decision was made to spare the first metatarsal from resection, despite the bony erosion. The pre-operative imaging characteristics and intraoperative imaging findings suggested that the lesion was caused by chronic mechanical pressure from the tumor and not from infiltration by the tumor per se. Other space-occupying lesions, such as rheumatoid nodules and aortic aneurysms have also demonstrated such a behavior on adjacent bone [6]. In these cases, bony erosion seems to be the result of bone resorption from biomechanical pressure, rather than bone destruction [7]. An alternative to the interoperative management would have been to add a core biopsy of the first metatarsal. After oncologic consultation and in accordance to general oncological principles dictated by the current literature open biopsy is preferable for musculoskeletal tumors and is shown to be more accurate compared to ultrasound guided fine needle and core biopsy [8]. In addition to this, due to the encircling nature of the primary tumor, there were concerns that this could create an iatrogenic path for local spread. The remodeling of the first metatarsal and absence of findings suggestive of a local recurrence eventually justified the choice to preserve the first ray. In any case, a ray amputation remains an option in the event of late recurrence involving the metatarsal. The incidence of such ray amputations in bony GCT tumors of the foot and ankle is reported to be as low as 11% [5]. Following oncologic consultation and in line with current literature, adjuvant therapy was not implemented in this case. Lately, targeted immunological therapies have been added to several treatment protocols. This came as a result of the identification of a common chromosomal abnormality in some of the tumor cells which leads to an over expression of colony-stimulating factor 1. Other adjuncts to operative intervention include external beam radiation therapy [9] and isotopic synovectomy [10, 11, 12]. At present, such a combined treatment, regime is mainly implemented in short-term recurrent disease [12] and its efficacy is yet to be validated [2, 12]. Recurrence rate of dt GCT remains considerably high. More than half of the recurrences occur in the first 2-year postoperatively, with an overall rate of 7% within the first 5 years [13]. In our case, the patient shows no signs of recurrence at the latest follow-up, 4 years postoperatively. She is scheduled for her final 5-year post-operative review in 2 months, after which time, she will be discharged.
The current case suggests that bone remodeling in the vicinity of diffuse type giant cell tumors is possible following a complete resection of the mass, provided that the erosion is caused by chronic mechanical pressure and there is no intraosseous expansion of the tumor.
The term diffuse type tenosynovial giant cell tumor indicate involvement of multiple tissues. The presence of bone lesions could raise concerns regarding the treatment options and the level of surgical intervention. If tumor spread in the adjacent bone is precluded, full resection of the tumor is shown to be adequate treatment, leading to bone remodeling in case of bony erosion caused by mechanical pressure.
References
- 1.Somerhausen NS, van den Rijn M. Tenosynovial giant cell tumor, diffuse type. In: Fletcher CD, Bridge JA, Hogendoorn PC, Mertens F, editors. World Health Organization Classification of Tumors. Pathology and Genetics of Tumors of Soft Tissue and Bone. 4th ed. Lyon: IARC Press; 2013. p. 102-3. [Google Scholar]
- 2.Fraser EJ, Sullivan M, Maclean F, Nesbitt A. Tenosynovial giant-cell tumors of the foot and ankle: A critical analysis review. JBJS Rev 2017;5: 01874474-201701000-00001. [Google Scholar]
- 3.Chou LB, Ho YY, Malawer MM. Tumors of the foot and ankle: Experience with 153 cases. Foot Ankle Int 2009;30:836-41. [Google Scholar]
- 4.Toepfer A, Harrasser N, Recker M, Lenze U, Pohlig F, Gerdesmeyer L, et al. Distribution patterns of foot and ankle tumors: A university tumor institute experience. BMC Cancer 2018;18:735. [Google Scholar]
- 5.Rajani R, Schaefer L, Scarborough MT, Gibbs CP. Giant cell tumors of the foot and ankle bones: High recurrence rates after surgical treatment. J Foot Ankle Surg 2015;54:1141-5. [Google Scholar]
- 6.Aydogan M, Karatoprak O, Mirzanli C, Ozturk C, Tezer M, Hamzaoglu A. Severe erosion of lumbar vertebral body because of a chronic ruptured abdominal aortic aneurysm. Spine J 2008;8:394-6. [Google Scholar]
- 7.Monsees B, Murphy WA. Pressure erosions: A pattern of bone resorption in rheumatoid arthritis. Arthritis Rheum 1985;28:820-4. [Google Scholar]
- 8.Traina F, Errani C, Toscano A, Pungetti C, Fabbri D, Mazzotti A, et al. Current concepts in the biopsy of musculoskeletal tumors: AAOS exhibit selection. J Bone Joint Surg Am 2015;97:e7. [Google Scholar]
- 9.Lee M, Mahroof S, Pringle J, Short SC, Briggs TW, Cannon SR. Diffuse pigmented villonodular synovitis of the foot and ankle treated with surgery and radiotherapy. Int Orthop 2005;29:403-5. [Google Scholar]
- 10.Bickels J, Isaakov J, Kollender Y, Meller I. Unacceptable complications following intra-articular injection of yttrium 90 in the ankle joint for diffuse pigmented villonodular synovitis. J Bone Joint Surg Am 2008;90:326-8. [Google Scholar]
- 11.Shabat S, Kollender Y, Merimsky O, Isakov J, Flusser G, Nyska M, et al. The use of surgery and yttrium 90 in the management of extensive and diffuse pigmented villonodular synovitis of large joints. Rheumatology (Oxford) 2002;41:1113-8. [Google Scholar]
- 12.Mastboom MJ, Palmerini E, Verspoor FG, Rueten-Budde AJ, Stacchiotti S, Staals EL, et al. Surgical outcomes of patients with diffuse-type tenosynovial giant-cell tumours: An international, retrospective, cohort study. Lancet Oncol 2019;20:877-6. [Google Scholar]
- 13.Siegel M, Bode L, Südkamp N, Kühle J, Zwingmann J, Schmal H, et al. Treatment, recurrence rates and follow-up of tenosynovial giant cell tumor (TGCT) of the foot and ankle-a systematic review and meta-analysis. PLoS One 2021;16:e0260795. [Google Scholar]