Gas gangrene is a rare condition, and hence, recognition of these uncommon presentations and timely diagnosis with a multidisciplinary approach are crucial to prevent complications and spread of the infection.
Dr. Jesmick Ponniah, Department of Orthopaedics, Velammal Medical College Hospital and Research Institute, Madurai, Tamil Nadu, India. E-mail: jesmickgarry@gmail.com
Introduction: We present a case of a 51-year-old gentleman admitted to the emergency department with a 2-day history of severe left forearm symptoms following a Gustillo-Anderson type II left both bone fracture sustained during a bicycle accident. Initially managed conservatively at an outside facility with an above-elbow backslab, his condition rapidly deteriorated, necessitating transfer to our institution. Adding complexity to his clinical presentation was his history of poorly controlled type 2 diabetes mellitus.
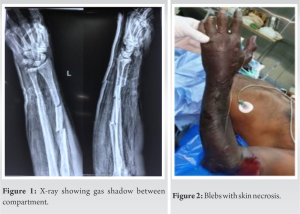
Case Report: On arrival, the patient exhibited fever and pronounced limb abnormalities, including extensive swelling, brownish skin with oozing blisters, and subcutaneous crepitus spanning from wrist to elbow. Radiological imaging unequivocally identified gas within the forearm compartments, signifying a severe infection. A provisional diagnosis of gas gangrene was made, later confirmed by culture report through intraoperative sampling.
Conclusion: Gas gangrene was definitively diagnosed, prompting immediate emergency surgery. Extensive necrosis was discovered during the procedure, necessitating an above-elbow amputation for limb salvage. Unfortunately, postoperatively, the infection continued to advance, extending to the chest and neck regions. Expert consultations in cardiothoracic and general surgery led to further extensive surgical interventions, including complete glenohumeral disarticulation and fasciotomy over chest for surgical emphysema. Microbiological analysis identified Clostridium perfringens, Escherichia coli, and Enterococcus as causative organisms. Aggressive antibiotic therapy, repeated surgical debridement, and meticulous wound management ultimately resulted in a successful recovery. The patient was discharged 20 days after initial presentation, underscoring the critical importance of prompt recognition and multidisciplinary management in severe gas gangrene cases, particularly in patients with underlying medical comorbidities.
Keywords: Oozing blisters, subcutaneous crepitus, extensive necrosis, compartment syndrome.
Gas gangrene or Clostridial myonecrosis is a necrotic infection of skin and soft tissue and it is characterized by the presence of gas under the skin which is produced by Clostridium. It is a potentially lethal disease which spreads quickly in soft tissues of the body. Tissue necrosis is due to production of exotoxins by spore forming gas producing bacteria in an environment of low oxygen. Gas gangrene is subclassified into two categories. Traumatic or post-operative is the most common form of gas gangrene accounting for 70% of the cases, followed by spontaneous or non-traumatic gangrene [1]. Clostridium perfringens is isolated in approximately 80% of patients presenting with traumatic gas gangrene, followed by Clostridium septicum, Clostridium novyi, Clostridium histolyticum, Clostridium bifermentans, Clostridium tertium, and Clostridium fallax. Herein, we report a case of gas gangrene which was treated with amputation, surgical debridement, and antibiotics.
A 51-year-old gentleman arrived at the emergency department with a 2-day history of significant left forearm swelling, pain, and discoloration. Four days prior, he experienced a bicycle accident resulting in a Gustillo-Anderson type II left both bone fracture. Initially managed with an above-elbow backslab at another facility, his condition progressively worsened, marked by increased pain, swelling, numbness, and loss of finger movement. Subsequently, he sought care elsewhere, where the back slab was removed, and a presumptive diagnosis of compartment syndrome was made before his transfer to our hospital. Notably, the patient had a history of poorly controlled type 2 diabetes mellitus for 8 years, managed solely with oral hypoglycemic drugs [2], without any other medication usage. On examination, he presented with fever (38.2°C), tachycardia (110 beats/min), and swelling accompanied by brownish skin and exuding bullae on the severely affected limb (Fig. 2). Subcutaneous crepitus extended along the forearm and discoloration spread from the wrist to the elbow joint. Radiographic imaging revealed gas within the forearm’s inter-facial planes [3], indicating extensive gas gangrene affecting all muscle compartments of the left forearm up to the elbow joint (Fig. 1). Further, investigations, including a CT chest, showed mediastinal involvement.
Diagnostic findings included a white blood cell count of 9400 cells/cu.mm, hemoglobin of 13.3 g/dL, an ESR of 84 mm, and an elevated procalcitonin level of 64.97, while CPK levels measured 1213. Urgent surgery was necessary due to extensive necrosis found on incision, leading to an above-elbow amputation as a life-saving measure. Post-surgery, signs of continued infection surfaced in the chest and neck regions, prompting consultations with cardiothoracic and general surgery. Further, emergency surgeries revealed necrotic tissue spread till the shoulder as shown in Fig. 3, necessitating a complete glenohumeral disarticulation and fasciotomy for surgical emphysema. Microbiological analysis confirmed C. perfringens, Escherichia coli, and Enterococcus as causative organisms. Aggressive antibiotic therapy (Injection Meropenem, injection Clindamycin, and injection Metrogyl) coupled with repeated surgical interventions, including wound debridement, led to a successful recovery. A subsequent wound exploration showed viable tissue without signs of spreading infection, allowing for closure and drain placement. Ultimately, the patient was discharged 20 days after initial presentation, emphasizing the critical role of prompt recognition and multidisciplinary management in severe gas gangrene cases, especially in patients with underlying medical comorbidities.
Gas gangrene is a severe, deadly anaerobic infection often associated with war. The occurrence of gas gangrene is to be feared in deep wounds complicated by impairment of the main or collateral circulation and especially by extensive crushing of muscle tissue associated with compound fractures. Gas gangrene is most frequently found in wounds of the lower extremity the period of invasion after trauma is 1–4 days, usually 24–48 h. The common pathogens of gas gangrene are Clostridium, are widely distributed in anaerobic nature, and are often found in soil putrilage as well as the digestive tract of animals and humans [4]. Clinically, gas gangrene is usually secondary to deep wounds contaminated with anaerobic Clostridiums, such as open fractures, lacerations, abrasions, and burns and may even appear in ulcers. Gas gangrene is often associated with impaired immune function and the patient usually has a combination of other diseases [5] such as uncontrolled diabetes mellitus and various forms of malignancies. In our case, the patient, who had a history of diabetes, initially received conservative care for the puncture wound, which might have contributed to the development of gas gangrene due to suboptimal wound management practices. The diagnosis of gas gangrene is mainly based on clinical symptoms and indications. The special sign of gas gangrene is pain in the region of a wound within a period of 1–4 days after injury. This pain is sudden in onset and frequently much more intense than the condition that the wound would indicate. The skin is edematous and darkened, and sometimes, tension blisters are visible around the wounds with a foul odor. There is a characteristic discharge with bubbles of gas, liquefied fat, and spreading crepitation that can be detected within a few hours. Meanwhile, the general condition of patients will deteriorate sharply and result in systemic toxemia, hypotension, shock, multi-organ failure, and death if not controlled. In managing gas gangrene, emphasizing early and swift diagnosis remains crucial. This allows for timely implementation of suitable treatments, encompassing supportive measures, antimicrobial therapy, and surgical intervention. The purpose of systemic supportive therapy is to maintain the stability of the circulatory volume and to improve the general condition of the patient. In the meantime, the use of antibiotics (penicillin, clindamycin, and metronidazole) [6, 7] is an important method to combat the effects of the infection. Hyperbaric oxygen therapy can also be considered to be an adjunctive measure, although its effectiveness has not yet been established [8]. Although many methods have been used to treat gas gangrene, surgery is still the most effective method. Surgical intervention includes extensive debridement and amputation, and the type of surgery depends on the affected region, extent of the infection, and experience of the surgeon. In general, if only one muscle or muscle group is affected or the infection is on the trunk, these regions should be opened by generous incisions and the subcutaneous tissue and fascia should be removed to reduce tension and establish a broad drainage with vacuum dressing [9]. On the other hand, if all of the muscles are affected on one extremity, amputation is imperative. The indication for amputation includes extreme lacerations of soft parts, the involvement of the infection on several groups of muscles, and the presence of extensive comminution of bone with or without opening into a large joint where gangrene is self-evident, and early development of symptoms of toxemia. Amputation is also indicated when the infection shows a tendency to extend rapidly toward the trunk. Sacrifice of the extremity is inevitable, it is important to assess the plane of amputation, which should be well above the infected area [10]. Thus, the patient started to improve once the infective foci was removed, that is, after glenohumeral disarticulation. All of the involved muscle tissue and suspected infected soft tissue should be excised, and the stump should be left open as shown in Fig. 4 to establish a broad drainage until the culture of the discharge from the granulating stump shows an absence of gas bacilli and the infection is under control.
Although gas gangrene is rare, the high mortality and disability due to gas gangrene force us to pay attention to its prophylaxis, and strict aseptic techniques should be emphasized for even the most minor procedure. For the treatment of gas gangrene, early and prompt diagnosis is critical, which can sometimes determine the patient outcome.
To enhance patient survival and reduce morbidity in gas gangrene, heightened vigilance is paramount when patients display symptoms such as infected tissue, necrosis, sepsis, or the presence of gas in affected areas. Early identification remains crucial, necessitating immediate surgical consultation for urgent debridement. Ensuring prompt transfer to specialized facilities capable of managing these critical cases.
References
- 1.Buboltz JB, Murphy-Lavoie HM. Gas Gangrene. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537030 [Last accessed on 2022 Aug 08]. [Google Scholar]
- 2.de Godoy JM, Godoy MF. Gas gangrene, diabetes and amputations of upper extremities. Acta Biomed 2020;91:44-6. [Google Scholar]
- 3.Ying Z, Zhang M, Yan S, Zhu Z. Gas gangrene in orthopaedic patients. Case Rep Orthop 2013;2013:942076. [Google Scholar]
- 4.Leiblein M, Wagner N, Adam EH, Frank J, Marzi I, Nau C. Clostridial gas gangrene ‐ a rare but deadly infection: Case series and comparison to other necrotizing soft tissue infections. Orthop Surg 2020;12:1733-47. [Google Scholar]
- 5.Wang S, Liu L. Gas gangrene following implant removal after the union of a tibial plateau fracture: A case report. BMC Musculoskelet Disord 2018;19:254. [Google Scholar]
- 6.Aldape MJ, Bayer CR, Rice SN, Bryant AE, Stevens DL. The comparative efficacy of antibiotics in treating experimental Clostridium septicum infection. Int J Antimicrob Agents 2018;52:469-73. [Google Scholar]
- 7.Stevens DL, Maier KA, Mitten JE. Effect of antibiotics on toxin production and viability of Clostridium perfringens. Antimicrob Agents Chemother 1987;31:213-8. [Google Scholar]
- 8.Gunter V. Gas gangrene treated by hyperbaric oxygen. Nurs Times 1969;65:526-8. [Google Scholar]
- 9.Liu Z, Zhao D, Wang B. Improved vacuum sealing drainage in the treatment of gas gangrene: A case report. Int J Clin Exp Med 2015;8:19626-8. [Google Scholar]
- 10.Zhu G, Zeng C, Yuan S, Li R. Emergency amputation necessitated within 24 hours by a human bite: A case report. J Int Med Res 2021;49:3000605211012201. [Google Scholar]