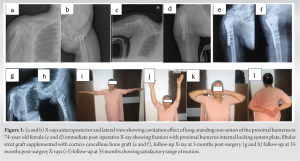
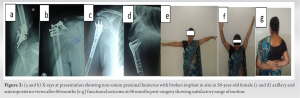
Poor quality bone in late presenting non-union of the proximal humerus can be successfully managed with adequate structural support using strut grafts and locking plates in addition to biological support.
Dr. Janki Sharan Bhadani, Consultant and DNB Appraiser, C/O Dr. John Mukhopadhaya, Director and HOD, Department of Orthopaedics, Paras HMRI Hospital Patna, Bihar, India. E-mail:jsbhadani@gmail.com
Introduction: It can be challenging to treat proximal humeral non-union (PHN). The challenge gets compounded when they are presented either late or after previous surgery. The challenges are far greater due to small proximal fragments, scalloping of the head, medial bone defect, osteoporosis, soft tissue contractures, and problems related to the previous implants.
Materials and Methods:In this retro-prospective study (2007–2020), we report on six cases of PHN which were presented to us more than 5 years after the original injury and managed using an intra-medullary autologous fibular strut graft (FSG) along with fixation with a proximal humeral locking plate and cancellous bone grafting. We quantified shoulder function based on constant score and disabilities of the arm, shoulder and hand (DASH) score.
Results: The mean age of patients is found to be 54.3 years (range, 22–74 years) with females dominating our study. The mean pre-operative constant score is 26.33 which improved to 71.83 in the post-operative period. The mean DASH score is 77.98 preoperatively, which improved to 19.5 postoperatively. The paired sample t-test compared the difference in mean of the pre-operative and post-operative scores, which shows significant improvement in outcome.
Conclusion: Even in very late PHN in poor-quality bone, the additional use of intramedullary strut grafts provides structural support to the fixation and further enhances the ability to withstand the load-start early motion and have a satisfactory functional outcome.
Keywords: Non-union, proximal humerus non-union, proximal humerus fracture, proximal humerus internal locking system, locking plate, autogenous fibular strut graft.
Proximal humerus non-union (PHN) is a highly crippling complication of proximal humerus fracture (PHF) and is associated with considerable morbidity. The rate of non-union after the closed treatment of PHF is estimated to be between 1.1% and 10% [1, 2]. The prevalence of PHN is higher in the presence of metaphyseal comminution, translation of the surgical neck, the interposition of the biceps tendon, deltoid muscle fibers or other soft tissue, synovial fluid at the fracture site, instability, inadequate fixation using a non-locking plate, post-operative infections, etc. [3, 4, 5, 6].
Challenges to managing such non-union include small proximal fragments, scalloping of the head, medial bone defect, osteoporosis, soft tissue contractures, and problems related to the previous implants, etc. Challenges are far greater if they present very late or after previous surgery [7]. PHN is more difficult to treat than non-union of other parts of the humerus [8]. Checchia et al., as well as Boileau et al., have proposed classification systems for PHN in different time zones, based on the location and complexity of the fracture [4, 9].
Different methods of osteosynthesis, implants, and arguments have been described with variable success rates, including inserting the shaft into the head, using an intramedullary cortical graft, blade plate, locking plate, and intramedullary nail including Polarus interlocking nail [10, 11, 12, 13]. Functional outcome of PHN using a locking plate or blade plate with or without bone graft is comparable [14, 15, 16, 17].
According to Ring et al., despite the longevity of the non-unions, it is possible to achieve healing using the basic concepts of the creation of a stable skeletal fixation in the presence of well-vascularized environments with the addition of autogenous bone graft [18]. Following the same concept, we report on six cases of PHN presented to us more than 5 years after the original injury, where we were able to save the humeral head and achieve satisfactory outcomes using an intra-medullary strut fibular autograft with proximal humerus internal locking system (PHILOS) and cancellous bone grafting.
We have done an observational, single-center, hospital-based retroprospective study in a referral center in eastern India to know the functional outcome of osteosynthesis of very late presenting PHN. We have collected data from January 2007 to December 2020 from the medical record section. Radiological and clinical photographs were collected from electronic records to evaluate non-union gaps, bone loss, type of non-union, etc. This study has received Institutional head and Ethical approval. Although thirty-one cases of PHN have been managed in our unit during this period, we included only those patients who met the following criteria. Those patients presented more than 5 years after failed conservative or operative management with poor quality of bone or after failed fixation, managed by osteosynthesis (autologous FSG, cancellous bone graft, and PHILOS plate). We have excluded those having screws penetrating the articular surface and have avascular necrosis (AVN). We found six such cases that fulfilled the inclusion criteria were included in the study. We interviewed, photographed, and assessed the patients during final follow-up visits.
The reasonable size of the proximal fragment is another point of interest defined by Boileau classification to decide whether it is possible to do osteosynthesis and salvage these or shoulder reconstruction could be considered.
All selected patients with PHN had large scalloped humeral head defects. We used the deltopectoral approach in all the patients as it is extensile and useful in case of later revision. Considering distorted anatomy, abundant scarring, poor bone quality, and varus deformity, the bone margin was freshened to achieve satisfactory bone alignment with no varus [19].(Fig.1) A bony defect in the head was estimated and fibular strut autograft (donor site: Ipsilateral leg) is positioned such that it provides medial support (Fig. 2).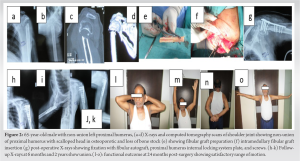
The flexibility and range of motion of the shoulder have been tested. The shoulder was immobilized in 30° abduction. After 2–3 days or after reduction of pain active pendulum exercise and passive abduction of the shoulder were started. Active abduction started after 4 weeks. In one case, there was severe abduction contracture, and she was kept in an abduction splint for 4 weeks before mobilization.
All patients were called for follow-up to evaluate clinically and radiologically at 6 weeks, 3 months, and every 6 months thereafter. The follow-up of patients varied between 30 and 102 months. The primary outcome variable is to determine shoulder function based on the constant score and disabilities of the arm, shoulder and hand (DASH) score during the final follow-up.
Secondary outcome variables are the demographic data of patients included in the study, factors that contributed to the initial non-union, the time of union, range of motion, and complications, as these factors can affect the functional outcome.
Paired sample t-test was used to compare the difference in mean of the pre-operative score and post-operative score of both the functional scoring systems for each patient included in the study. Mean standard deviation (SD) and standard error (SE) were calculated. We have kept the pre-operative and post-operative score as pair one for the constant score and the pre-operative and post-operative of the DASH score as pair two. Paired samples to score correlations were calculated separately for pair one and pair two.
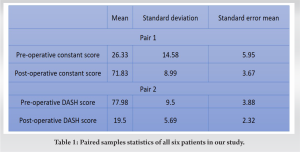
The clinical and functional results have been assessed at the time of the last follow-up. We are using the constant scoring system and DASH score to assess the functional outcome. The constant score consists of four variables including subjective factors (pain and the activities of daily living) and objective variables (range of motion etc.). DASH score ranges from zero (no disability) to 100 (most severe disability). The mean pre-operative constant score is 26.33 (range: 10–47) which improved to 71.83 (range: 57–83) in the post-operative period. A 10-point difference in mean DASH score may be considered a minimal important change. The mean DASH score is 77.98 (range: 61.6–87.1) preoperatively, which is improved to 19.5 (range 13.8–26.7) (Table 1) postoperatively. Thus, all patients have a greater than 10 points difference which is significant for the patient showing treatment effectiveness after surgery. [20].
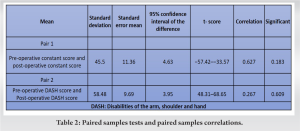
Paired sample t-test compared the difference in mean of the pre-operative score and post-operative score, which shows significant improvement postoperatively. The constant score and DASH score of pair 1 show SD of ±14.5 and 8.9 and the SE mean are 5.9 and 3.6. Pair 2 has ±SD 9.5/5.6 and SE means 3.8/2.32. The correlation between pair 1 having a value of 0.627 and pair 2 having 0.267 (Table 2). Correlation describes the association between two variables. The pre-operative constant scores increased postoperatively while the pre-operative DASH scores decreased postoperatively. The difference between the scores (pre- and post-operative) was statistically significant (P = 0.000 in both).
We have used descriptive statistics to report the outcome of six patients including two males and four females, between 22 and 74 years of age (Table 3).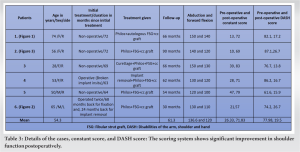
Union was achieved in 6 months (mean). The duration of union time was calculated from the first presentation. Where fracture united in the X-ray. The union was achieved earlier in those, who initially managed conservatively (mean union time 5.5 months) than in those, who were initially managed operatively (mean union time 7 months). There was no significant pain or disability in our study. However, one patient developed discomfort, occasional pain, and restriction of overhead abduction due to hardware prominence, for which implant removal was done at 2-year follow-up. The average abduction achieved was 136.6° (range: 120–150°) and the average forward flexion achieved was 120° (range: 100–140°).
PHN is associated with considerable morbidity including pain and stiffness, in association with shoulder dysfunction in most [21]. There are no guidelines available to manage very late presenting PHN. No single implant is found to be ideal. In this study, we assessed the functional outcome of such cases managed with a locking plate, autologous fibular strut, and cancellous graft.
Outcome
In our study, mean constant score was improved to 71.83 (the pre-operative score was 26.33), which is comparable to Allende and Allende (72.7), Gao et al. (77.7), and Quadlbauer et al. (71.0) [22, 23, 24]. Ring et al., Allende and Allende, and Healy et al. also achieved 92–100% union in similar cases treated with plate and bone grafting [14, 22, 26] though Quadlbauer et al. (9 cases) and Tauber et al. (55 cases) had been done osteosynthesis using plate but without bone graft and achieved a similar result (93% union and 100%, respectively) [24, 32].
Demographic data
The mean age of the patients in our study is 54.3 years (Table 3). This is comparable to the Ahrens et al. study (56.2 years), which treated five such non-union using autologous FSG and locking plate and achieved a satisfactory outcome in 80% of the cases [21]. Our study has a female-predominant population (M:F=2:4) like most of the studies, e.g.: Badman et al. (0:18), Hettrich et al. (0:3), Ahrens et al. (1:4), Allende and Allende (0:7), Gao et al. (2:5), Quadlbauer et al. (3:6) [16, 17, 21, 22, 23, 24].
Risk factors
The factors that contributed to the initial non-union could potentially lead to a treatment failure when addressing the non-union with open reduction and initial fixation (ORIF). Smoking is a well-known risk factor for PHN [2, 25, 26]. Sanchez described those younger patients who developed non-union usually and had higher Elixhauser-Comorbidity Index scores [25]. Two (33%) patients were smokers in this study. Rollo et al. found that 50% (8 out of 16) patients were smokers and two were addicted to alcohol [27]. Hanson et al., found that smokers have 5.5 times more risk of developing non-union [2]. Among four female patients, three had iron deficiency anemia, and two had hypertension.
Surgical techniques
Healy et al. found a better outcome of treatment, after open reduction with internal fixation and bone grafting when compared with other methods of treatment including un-reamed intramedullary fixation and proximal humeral hemiarthroplasty [26].
Implants and augments
There are multiple types of implants and augments to manage PHN with variable success rates. Ring et al. [14] reported that, 80% satisfactory outcome and 92% union among 25 PHN treated with a blade plate and autogenous bone grafting healed. Galatz et al. [15] 100% satisfactory outcome (100%) treated with either blade plate and bone graft (10 cases) or T-plate with bone graft. Badman et al. treated 18 patients with fibular allograft and the fixed-angle device showed union in 17 patients [16]. However, the ideal treatment for this disabling condition remains unclear and largely depends on the judicious use of treatment methods and surgical expertise [17]. We have noticed scalloping of the humeral head, bone gap, and local (or generalized) osteoporosis in all the cases, probably due to late presentation. End-to-end alignment of the fragments usually does not provide good bone contact and stability for these fractures. The autogenous intramedullary FSG helps obliterate the cavitation (scalloping) of the humeral head fragment, substitutes for bone stock deficiency, gives medial structural, and endosteal support, and avoids varus. Georgiadis and Georgiadis have used an intramedullary tantalum cylinder instead of FSG to provide endosteal support [28]. We have used a lateral PHILOS plate, through which some screws have been applied through FSG to enhance the strength of fixation. Other than locking plates and FSG, we also used cancellous autograft, which has provided improved biomechanical stability [24, 29].
A longer plate has been suggested for those PHN that was initially managed with a plate and failed. A longer plate increases the working length and provides a stable fracture-healing environment [9]. We have successfully managed those two patients with longer locked plates, who were managed initially with a plate. There was a relatively higher rate of reoperation in the ORIF group compared to the hemiarthroplasty group for several causes, including adhesion, lyses, loss of instrumentation, or ongoing non-union [13]. There was no need for reoperation in any of the six cases in our study.
Union time
Union was achieved in all patients at a mean of 6 months in our study. This is comparable to similar studies done by Badman et al. (5.4 months), Allende and Allende (5.9 months), Gao et al. (6.1 months), and Carlock et al. (5.4 months) [16, 22, 23, 30]. Badman et al. achieved union in 17 out of 18 non-unions [16]. Quadlbauer et al. concluded that locking plate fixation without bone grafting is a reasonable and safe option for treating proximal humerus non-union. However, Zastrow et al. found lesser union time when used with autograft [24, 29]. The union time of PHN using a locked plate augmented with autograft and found shorter (3.9 months) for those managed conservatively for original fracture and more for those managed operatively (5.5 months) for original fracture [29]. In our study, the union was achieved early in those, who initially managed conservatively (mean union time 5.5 months) than in those, who were initially managed operatively (mean union time 7 months).
Complications
Carlock et al. had shown a few complications such as one AVN and two cases (12.5%) of hardware failure requiring reoperation in the non-union group of patients [30]. Ring et al. and Tauber et al. also reported similar complications up to 15% [14, 31]. Though no such complications occurred in our study. There was discomfort, occasional pain, and restriction of overhead abduction due to hardware prominence in one patient. One of the five patients in the Ahrens et al. study had developed donor site morbidity, in his study (sensory changes in the area of superficial peroneal nerve improved in 6 weeks) and failure of fixation in 15 months [21]. In our study, we have noticed no donor site morbidity in any case.
FSG was first explained by Gardner et al. for medial column support [32]. Increased use of FSG in fresh fracture is gaining popularity, with a high grade of success, in the presence of comminution and osteoporosis [33, 34, 35]. We have used autologous FSG in all PHN. The subset of six patients from the study done by Carlock et al. also used FSG in six cases of PHN. But, unlike our study, the author used allograft, which might be the reason for one early fixation failure and AVN. Although the author agrees that graft type has an effect on the outcomes, disagrees that these complications were related to the allograft [30]. Barnes et al. also used fibular strut allograft, only 1 of 3 patients of proximal humerus non-union did reach union [36]. Literature supports the use of fibular strut allograft in the case of hypertrophic non-union for additional mechanical support as biology is preserved in such cases [36, 37, 38, 39, 40].
Strength and limitations
Our study is probably the first of its kind where we have selected only those cases of PHN that was presented 5 years after the initial fracture and managed by osteosynthesis. Due to the rarity of such cases, the small number of patients over a large period is one of the limitations of our study. We included a heterogeneous group of patients who were initially managed either by conservative or operative treatment, which is another shortcoming of our study. Another limitation of this study is that the duration of union time was calculated from the first presentation, where the fracture united in the X-ray.
Symptomatic PHN can be managed operatively even in those presented very late. A locking plate alone is not sufficient due to the corresponding bony gap, scalloping of the humeral head, etc. We can achieve union and satisfactory functional outcomes using PHILOS with augmentation of autologous FSG and cancellous graft. A prospective, multicentric study with many such cases will increase the efficacy and reliability of the study.
Osteoporotic late presenting proximal humerus non-union should take into account mechanical support as well as biology using FSG and autologous cancellous graft, respectively. Both the above-mentioned types of grafts have proven useful in addition to fixation with fixed angle devices (locking plate/PHILOS) for successful outcomes of osteosynthesis.
References
- 1.Cadet ER, Yin B, Schulz B, Ahmad CS, Rosenwasser MP. Proximal humerus and humeral shaft nonunions. J Am Acad Orthop Surg 2013;21:538-47. Erratum in: J Is Acad Orthop Surg 2013;21:21a. [Google Scholar]
- 2.Hanson B, Neidenbach P, de Boer P, Stengel D. Functional outcomes after nonoperative management of fractures of the proximal humerus. J Shoulder Elbow Surg 2009;18:612-21. [Google Scholar]
- 3.Court-Brown CM, McQueen MM. Nonunions of the proximal humerus: Their prevalence and functional outcome. J Trauma 2008;64:1517-21. [Google Scholar]
- 4.Checchia SL, Doneux P, Miyazaki AN, Spir IA, Bringel R, Ramos CH. Classification of non-unions of the proximal humerus. Int Orthop 2000;24:217-20. [Google Scholar]
- 5.Walch G, Badet R, Nové-Josserand L, Levigne C. Nonunions of the surgical neck of the humerus: Surgical treatment with an intramedullary bone peg, internal fixation, and cancellous bone grafting. J Shoulder Elbow Surg 1996;5:161-8. [Google Scholar]
- 6.Poelmann J, Kloen P. Modified use of the proximal humeral internal locking system (PHILOS) plate for distal femoral nonunions. Eur J Orthop Surg Traumatol 2023;33:425-33. [Google Scholar]
- 7.Athwal GS, Sperling JW, Rispoli DM, Cofield RH. Acute deep infection after surgical fixation of proximal humeral fractures. J Shoulder Elbow Surg 2007;16:408-12. [Google Scholar]
- 8.Fink Barnes LA, Ruig FH, Freibott CE, Rajfer R, Rosenwasser MP. Treatment of nonunions of the humeral shaft with non vascularized fibular strut allograft: Postoperative outcomes and review of a surgical technique. JSES Int 2020;4:739-44. [Google Scholar]
- 9.Boileau P, Trojani C, Walch G, Krishnan SG, Romeo A, Sinnerton R. Shoulder arthroplasty for the treatment of the sequelae of fractures of the proximal humerus. J Shoulder Elbow Surg 2001;10:299-308. [Google Scholar]
- 10.Tapscott DC, Paxton ES. Decision-making and management of proximal humerus nonunions. Orthop Clin North Am 2021;52:369-79. [Google Scholar]
- 11.Martinez AA, Bejarano C, Carbonel I, Iglesias D, Gil-Albarova J, Herrera A. The treatment of proximal humerus nonunions in older patients with reverse shoulder arthroplasty. Injury 2012;43 Suppl 2:S3-6. [Google Scholar]
- 12.Hamilton SW, Baird KS. The treatment of established non-union of the proximal humerus using the Polarus locking intramedullary nail. Int J Shoulder Surg 2009;3:53-6. [Google Scholar]
- 13.Duralde XA, Flatow EL, Pollock RG, Nicholson GP, Self EB, Bigliani LU. Operative treatment of nonunions of the surgical neck of the humerus. J Shoulder Elbow Surg 1996;5:169-80. [Google Scholar]
- 14.Ring D, McKee MD, Perey BH, Jupiter JB. The use of a blade plate and autogenous cancellous bone graft in the treatment of ununited fractures of the proximal humerus. J Shoulder Elbow Surg 2001;10:501-7. [Google Scholar]
- 15.Galatz LM, Williams GR Jr., Fenlin JM Jr., Ramsey ML, Iannotti JP. Outcome of open reduction and internal fixation of surgical neck nonunions of the humerus. J Orthop Trauma 2004;18:63-7. [Google Scholar]
- 16.Badman BL, Mighell M, Kalandiak SP, Prasarn M. Proximal humeral nonunions treated with fixed-angle locked plating and an intramedullary strut allograft. J Orthop Trauma 2009;23:173-9. [Google Scholar]
- 17.Hettrich CM, Paul O, Neviaser AS, Borsting EA, Lorich DG. The anterolateral approach to the proximal humerus for nonunions and delayed unions. Int J Shoulder Surg 2011;5:21-5. [Google Scholar]
- 18.Ring D, Barrick WT, Jupiter JB. Recalcitrant nonunion. Clin Orthop Relat Res 1997;340:181-9. [Google Scholar]
- 19.Banco SP, Andrisani D, Ramsey M, Frieman B, Fenlin JM Jr. The parachute technique: Valgus impaction osteotomy for two-part fractures of the surgical neck of the humerus. J Bone Joint Surg Am 2001;83-A:38-42. [Google Scholar]
- 20.Gummesson C, Atroshi I, Ekdahl C. The disabilities of the arm, shoulder and hand (DASH) outcome questionnaire: Longitudinal construct validity and measuring self-rated health change after surgery. BMC Musculoskelet Disord 2003;4:11. [Google Scholar]
- 21.Ahrens PM, Nicoletti S, Houghton-Clemmey RS. Nonunion of the surgical neck of the humerus: Treatment with fibular graft and locking plate fixation. Tech Shoulder Elbow Surg 2010;11:15-8. [Google Scholar]
- 22.Allende C, Allende BT. The use of a new locking 90-degree blade plate in the treatment of atrophic proximal humerus nonunions. Int Orthop 2009;33:1649-54. [Google Scholar]
- 23.Gao K, Gao W, Huang J, Wu X, Wang CS, Wang Q. Treatment of surgical neck nonunions of the humerus with locked plate and autologous fibular strut graft. Med Princ Pract 2012;21:483-7. [Google Scholar]
- 24.Quadlbauer S, Hofmann GJ, Leixnering M, Rosenauer R, Hausner T, Reichetseder J. Open reduction and fixation with a locking plate without bone grafting is a reasonable and safe option for treating proximal humerus nonunion. Int Orthop 2018;42:2199-209. [Google Scholar]
- 25.Sanchez G, Vargas M, Gordon AM, Golub I, Ashraf A, Diamond K, et al. Risk factors for nonunion following open reduction and internal fixation for proximal humerus fractures. Eur J Orthop Surg Traumatol 2022;33:883-8. [Google Scholar]
- 26.Healy WL, Jupiter JB, Kristiansen TK, White RR. Nonunion of the proximal humerus. A review of 25 cases. J Orthop Trauma 1990;4:424-31. [Google Scholar]
- 27.Rollo G, Rotini R, Pichierri P, Giaracuni M, Stasi A, Macchiarola L, et al. Grafting and fixation of proximal humeral aseptic non union: A prospective case series. Clin Cases Miner Bone Metab 2017;14:298-304. [Google Scholar]
- 28.Georgiadis GM, Georgiadis AG. Proximal humeral nonunion treated with an intramedullary tantalum cylinder. Orthopedics 2013;36:e1555-8. [Google Scholar]
- 29.Zastrow RK, Patterson DC, Cagle PJ. Operative management of proximal humerus nonunions in adults: A systematic review. J Orthop Trauma 2020;34:492-502. [Google Scholar]
- 30.Carlock KD, Konda SR, Bianco IR, Zuckerman JD, Egol KA. Repair of proximal humerus fracture nonunions using a standardized treatment algorithm: A case series. Eur J Orthop Surg Traumatol 2021;31:1151-9. [Google Scholar]
- 31.Tauber M, Brugger A, Povacz P, Resch H. Reconstructive surgical treatment without bone grafting in nonunions of humeral surgical neck fractures. J Orthop Trauma 2011;25:392-8. [Google Scholar]
- 32.Gardner MJ, Boraiah S, Helfet DL, Lorich DG. Indirect medial reduction and strut support of proximal humerus fractures using an endosteal implant. J Orthop Trauma 2008;22:195-200. [Google Scholar]
- 33.Panchal K, Jeong JJ, Park SE, Kim WY, Min HK, Kim JY, et al. Clinical and radiological outcomes of unstable proximal humeral fractures treated with a locking plate and fibular strut allograft. Int Orthop 2016;40:569-77. [Google Scholar]
- 34.Neviaser AS, Hettrich CM, Beamer BS, Dines JS, Lorich DG. Endosteal strut augment reduces complications associated with proximal humeral locking plates. Clin Orthop Relat Res 2011;469:3300-6. [Google Scholar]
- 35.Tan E, Lie D, Wong MK. Early outcomes of proximal humerus fracture fixation with locking plate and intramedullary fibular strut graft. Orthop 2014;37:e822-7. [Google Scholar]
- 36.Berkes MB, Little MT, Lazaro LE, Cymerman RM, Pardee NC, Helfet DL, et al. Intramedullary allograft fibula as a reduction and fixation tool for the treatment of complex proximal humerus fractures with diaphyseal extension. J Orthop Trauma 2014;28:e56-64. [Google Scholar]
- 37.Mathison C, Chaudhary R, Beaupre L, Reynolds M, Adeeb S, Bouliane M, et al. Biomechanical analysis of proximal humeral fixation using locking plate fixation with an intramedullary fibular allograft. Clin Biomech (Bristol, Avon) 2010;25:642-6. [Google Scholar]
- 38.Chow RM, Begum F, Beaupre LA, Carey JP, Adeeb S, Bouliane MJ. Proximal humeral fracture fixation: Locking plate construct ± intramedullary fibular allograft. J Shoulder Elbow Surg 2012;21:894-901. [Google Scholar]
- 39.Little MT, Berkes MB, Schottel PC, Lazaro LE, LaMont LE, Pardee NC, et al. The impact of preoperative coronal plane deformity on proximal humerus fixation with endosteal augmentation. J Orthop Trauma 2014;28:338-47. [Google Scholar]
- 40.Matassi F, Angeloni R, Carulli C, Civinini R, Di Bella L, Redl B, et al. Locking plate and fibular allograft augmentation in unstable fractures of proximal humerus. Injury 2012;43:1939-42. [Google Scholar]