This case report serves as a reminder to health-care professionals to consider fungal peri-prosthetic joint infection in their differential diagnosis, even in patients without apparent risk factors, and to emphasize the importance of timely diagnosis and appropriate treatment.
Dr. Meena Mishra, Department of Microbiology, All India Institute of Medical Sciences, Nagpur, Maharashtra, India/Staff Quarters, All India Institute of Medical Sciences, Nagpur, MIHAN, Nagpur - 441 108, Maharashtra, India. E-mail: meenamishra@aiimsnagpur.edu.in
Introduction: The recent surge in joint replacement surgeries in India, particularly total knee and hip replacements, is notable. The majority of patients undergoing these procedures suffered from knee osteoarthritis or femoral head avascular necrosis. However, this increase in joint replacements has also led to a rise in periprosthetic joint infections (PJI), a severe and costly complication. PJI is responsible for 25% of total knee arthroplasty failures and 15% of total hip arthroplasty failures. Various risk factors, such as diabetes, fractures, and arthritis, are associated with PJI development. Bacterial infections, notably Staphylococcus species, are the primary cause of PJI, but rare fungal infections are often caused by Candida and Aspergillus. There is a lack of clinical trials and limited knowledge about the prevalence and treatment of fungal PJIs.
Case Report: This report presents a unique case of delayed PJI caused by Candida albicans in an otherwise healthy patient.
Conclusion: This case report emphasizes the need for further research and standardized treatment guidelines. Treatment typically involves a two-stage revision with extended antifungal therapy. The optimal duration of antifungal treatment remains uncertain.
Keywords: Joint replacement surgeries, periprosthetic joint infections, fungal periprosthetic joint infections, Candida albicans, two-stage revision arthroplasty.
In recent times, India has experienced a substantial increase in joint replacement surgeries, specifically total knee and hip replacements. The number of cases has risen from 1,019 in 2006 to 27,000 in 2019 [1]. A significant majority of these patients (98.5%) were suffering from knee joint osteoarthritis or avascular necrosis of the femoral head [1]. Periprosthetic joint infection (PJI) stands out as one of the most severe and expensive complications that can occur after total joint arthroplasty (TJA). PJI is responsible for approximately 25% of failures in total knee arthroplasty and 15% of failures in total hip arthroplasty (THA) [2]. As the prevalence of joint arthroplasties continues to rise, the incidence of PJI is expected to increase, which will present substantial challenges for both patients and the health-care system. Various risk factors have been associated with the development of PJI, including conditions such as diabetes mellitus, avascular necrosis of the femoral head, femoral neck fractures, rheumatoid arthritis, cardiovascular disease, and osteoarthritis [3]. Bacterial infections, primarily Staphylococcus aureus and coagulase-negative staphylococci, are the main culprits behind PJI, making up 50–60% of cases [4]. Although fungal infections are rare, they can be devastating, often caused by Candida and Aspergillus species [5]. Managing fungal PJIs is particularly challenging due to the complex structure of fungal biofilms and the lack of established treatment guidelines [6]. Because fungal PJIs are so uncommon, there is a scarcity of clinical trials and limited knowledge regarding their prevalence, risk factors, sources, and treatment protocols. Therefore, case reports of fungal PJIs play a crucial role in improving our understanding of this condition. This report details a unique case of delayed PJI caused by Candida albicans in an immunocompetent patient without underlying comorbidities, a scenario rarely documented in Central India.
A 33-year-old female patient sought treatment in our orthopedic department, complaining of right hip pain. Her history included a fall and hip injury over a decade ago, which had been managed conservatively, providing relief. However, 2 years later, she began experiencing recurrent hip pain without recent trauma, leading to sporadic use of analgesics over 5 years. She visited a local orthopedic hospital where she was diagnosed with right hip arthritis secondary to post-traumatic avascular necrosis of the femoral head according to her symptoms and radiological findings (Fig. 1). In 2018, she underwent a right THA outside our hospital for hip joint arthritis. Postoperatively, she experienced a right hip dislocation on the 3rd day, which was reduced at the same facility under spinal anesthesia. A second hip dislocation occurred on the 12th day, followed by another reduction and 15 days of immobilization with a Thomas splint. After 15 days, partial weight-bearing mobilization with a walker began, and she was discharged a few days later. Three months later, she progressed to full weight-bearing mobilization with intermittent analgesic use. Despite no fresh trauma or pus discharge from the hip, she developed hip pain, leading to a recommendation for an ultrasound-guided biopsy, which showed no bacterial growth.
Four years post-THA, the patient presented to our hospital with progressively worsening right hip pain, difficulty walking, tenderness, local erythema, elevated local temperature, and restricted hip joint movement. Radiography revealed right cemented hip total hip joint replacement with loosened acetabular components (Fig. 2). While C-reactive protein (CRP) levels were normal, the erythrocyte sedimentation rate (ESR) was elevated at 100 mm/h. Ultrasound-guided aspiration from the right hip yielded no growth on aerobic culture. Subsequently, the patient underwent Stage 1 revision in which debridement, prosthesis removal, and antibiotic (vancomycin) cement spacer insertion were done (Fig. 3).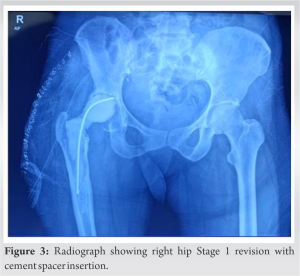
The patient was then started on intravenous amphotericin B and fluconazole for the next 2 weeks. After 14 days, suture removal revealed a dry and healthy wound. She was discharged on oral fluconazole 400 mg BD and Cefuroxime 500 mg BD and advised non-weight-bearing mobilization with a walker. Subsequent follow-ups were uneventful, and oral fluconazole continued for 4 weeks. The surgical wound healed well. After 3 months, she underwent cement spacer removal and second-stage revision total hip replacement without complications. The patient was discharged on oral cefuroxime 500 mg OD for 14 days and full weight-bearing mobilization with a walker. She remained asymptomatic during follow-up, 12 months after the revision hip joint replacement with no radiological evidence of implant loosening (Fig. 6).
Fungal PJIs are uncommon yet complex issues, typically affecting individuals who are immunocompromised, have underlying health conditions, or have been on extended antibiotic treatments [7]. This case study sheds light on a rare instance of PJI caused by C. albicans in a patient who is immunocompetent and lacks any predisposing factors. Fungal PJIs typically manifest as pain and swelling, and their symptoms can develop gradually. Because the symptoms are often mild, and Candida-related PJI is not typically suspected, the diagnosis is frequently delayed [7]. C. albicans is the most common fungal pathogen responsible for PJI, and it can occur either shortly after joint replacement surgery or much later, making it challenging to determine the source of the infection [5]. The diagnostic process for fungal PJIs can be complicated by normal or only slightly elevated inflammatory markers such as ESR and CRP. Identifying the presence of Candida in samples, especially when it is not detected in Gram-stained smears, can be challenging. To enhance sensitivity, it is recommended to use extended culture periods and blood culture bottles for synovial fluid analysis [7]. Treatment for fungal PJIs usually involves a two-stage revision arthroplasty accompanied by an extended course of antifungal therapy [6]. The choice of antifungal medication depends on local resistance patterns, with amphotericin B and fluconazole being effective options. Prolonged post-revision antifungal therapy is crucial, although the optimal duration is still uncertain [6]. Various factors contribute to the likelihood of fungal infections. Among these, factors related to the host’s health play a significant role. Conditions such as diabetes, weakened immune systems (either due to immunosuppression or immunodeficiency), rheumatoid arthritis, cancer, tuberculosis, and issues related to kidney function or transplantation have been linked to an increased risk of fungal infections [8, 9]. In addition, the body’s immune response and nutritional health also play a crucial role in determining the susceptibility to these infections [10]. However, this was a rare case in which a nutritionally fair and immunocompetent patient developed fungal PJI without any apparent risk factors.
Fungal PJIs are rare but intricate complications of joint arthroplasties. This case underscores the importance of timely microbiological diagnostics and two-stage revision with extended antifungal therapy in managing such infections. Fungal PJIs should be considered even in immunocompetent patients without apparent risk factors. Further research and standardized treatment guidelines are needed to enhance our understanding and management of fungal PJIs.
Clinicians should maintain a high index of suspicion for fungal PJIs, as they can occur even in immunocompetent patients without known risk factors. Fungal PJIs may present with mild, gradual symptoms, delaying diagnosis. Utilizing extended culture methods and blood culture bottles for synovial fluid analysis can improve sensitivity in detecting fungal pathogens. Treatment typically involves a two-stage revision and extended antifungal therapy based on local resistance patterns. Given the scarcity of clinical trials and limited knowledge, further research is crucial to establish standardized guidelines for managing fungal PJIs. Early diagnosis and appropriate treatment are essential for improved outcomes in these challenging cases.
References
- 1.Vaidya SV, Jogani AD, Pachore JA, Armstrong R, Vaidya CS. India joining the world of hip and knee registries: Present status-A leap forward. Indian J Orthop 2020;55(Suppl 1):46-55. [Google Scholar]
- 2.Bonanzinga T, Tanzi P, Iacono F, Ferrari MC, Marcacci M. Periprosthetic knee infection: Two stage revision surgery. Acta Biomed 2017;88(Suppl 4):114-9. [Google Scholar]
- 3.Ren X, Ling L, Qi L, Liu Z, Zhang W, Yang Z, et al. Patients’ risk factors for periprosthetic joint infection in primary total hip arthroplasty: A meta-analysis of 40 studies. BMC Musculoskelet Disord 2021;22:776. [Google Scholar]
- 4.Tornero E, García-Oltra E, García-Ramiro S, Martínez-Pastor JC, Bosch J, Climent C, et al. Prosthetic joint infections due to Staphylococcus aureus and coagulase-negative staphylococci. Int J Artif Organs 2012;35:884-92. [Google Scholar]
- 5.Chisari E, Lin F, Fei J, Parvizi J. Fungal periprosthetic joint infection: Rare but challenging problem. Chin J Traumatol 2022;25:63-6. [Google Scholar]
- 6.Gross CE, Valle CJ, Rex JC, Traven SA, Durante EC. Fungal periprosthetic joint infection: A review of demographics and management. J Arthroplasty 2021;36:1758-64. [Google Scholar]
- 7.Koutserimpas C, Naoum S, Giovanoulis V, Raptis K, Alpantaki K, Dretakis K, et al. Fungal periprosthetic hip joint infections. Diagnostics 2022;12:2341. [Google Scholar]
- 8.Azzam K, Parvizi J, Jungkind D, Hanssen A, Fehring T, Springer B, et al. Microbiological, clinical, and surgical features of fungal prosthetic joint infections: A multi-institutional experience. J Bone Joint Surg Am 2009;91(Suppl 6):142-9. [Google Scholar]
- 9.Anagnostakos K, Kelm J, Schmitt E, Jung J. Fungal periprosthetic hip and knee joint infections clinical experience with a 2-stage treatment protocol. J Arthroplasty 2012;27:293-8. . [Google Scholar]
- 10.Kelesidis T, Tsiodras S. Candida albicans prosthetic hip infection in elderly patients: Is fluconazole monotherapy an option? Scand J Infect Dis 2010;42:12-21 [Google Scholar]














