It is imperative to consider tumor-induced osteomalacia as a potential cause for patients experiencing muscle weakness and pain. Swiftly locating and removing the tumor is paramount in relieving symptoms and restoring bone health.
Dr Shubham Tungenwar, Department of Orthopaedics, T N Medical College and BYL Nair Ch Hospital, Mumbai, Maharashtra, India. E-mail: tungenwarshubham@gmail.com
Introduction: An uncommon medical disorder known as tumor-induced osteomalacia (TIO) is characterized by severe hypophosphatemia, renal phosphate wasting, and osteomalacia due to a tumor. TIO has recently been linked to a particular kind of tumor known as phosphaturic mesenchymal tumor (PMT). PMTs release phosphatonins, such as fibroblast growth factor-23 (FGF23), which elevates serum levels of FGF23, leading to phosphate wasting and osteomalacia. However, due to their infrequent occurrence and vague symptoms, such as bone pain, myopathies, arthralgias, fractures, and weakness, the diagnosis of PMTs is often delayed or misdiagnosed. In this case report, a rare case of PMT in the proximal femur resulted in TIO, and it highlights the long and difficult journey from symptom onset to correct diagnosis and successful surgical management.
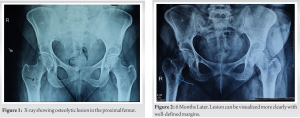
Case Report: A 51-year-old woman endured persistent joint pain, muscle weakness, and fatigue for 2 years. Despite having no known health issues, she suffered from hip pain that spreads to her knees and ankles, and tingling and paresthesia in her legs, making it difficult to bear weight. She underwent surgery to remove a parathyroid adenoma, but unfortunately, her symptoms returned. Her magnetic resonance imaging revealed a lesion in her proximal femur, which was promptly removed. The tissue examination results verified the identity of the tumor as a PMT. The patient’s phosphorus levels returned to normal and after a year of follow-up, she was able to resume normal daily activities, bear weight on the affected limb and showed no signs of the tumor recurrence.
Conclusion: Adult patients experiencing bone pain, progressive weakness, and multiple fractures with no family history of similar conditions should consider TIO as a potential cause. It is rare and often misdiagnosed and complete surgical removal of the tumor is the optimal treatment for TIO, resulting in the resolution of long-standing symptoms and biochemical abnormalities. Timely recognition, localization, and surgical removal of the tumor are crucial for symptom resolution and the restoration of normal bone mineralization.
Keywords: Hypophosphatemia, fibroblast growth factor 23, phosphaturic mesenchymal tumor, tumor-induced osteomalacia.
The unusual condition known as phosphaturic mesenchymal tumors (PMT) induces paraneoplastic tumor-induced osteomalacia (TIO). This condition is characterized by the release of phosphatonins, particularly fibroblast growth factor 23 (FGF23). FGF23, in turn, reduces the reabsorption of phosphate in the proximal renal tubules and inhibits the production of 1,25-dihydroxycholecalciferol by 1-α-hydroxylase [1]. These tumors are often small and challenging to identify during physical examination. Symptoms of osteomalacia, such as bone pain and generalized muscle weakness, typically precede the tumor diagnosis by several years, leading to significant morbidity [2, 3]. While cases of PMT are primarily reported in individual case studies, the imaging features of these tumors vary widely. Once diagnosed, complete resection with wide margins is typically performed to prevent local recurrence. This approach usually leads to rapid resolution of laboratory abnormalities and symptoms and is often considered curative [4].
We present the case of a 51-year-old female who complained of multiple joint pain, fatigue, and muscle weakness, persisting for 2 years. The patient had no known co-morbidities and initially experienced bilateral hip pain, gradually extending to both knees and ankles. In addition, she reported tingling and paresthesia in both lower limbs, making it difficult to bear weight. The patient was referred to the endocrinology department for evaluation because of concerns over bone fragility. There were no reports of falls, kidney stones, hereditary osteoporosis, or metabolic bone disease throughout her medical history. In addition, she denied ever having smoked, drunk alcohol, used steroids, or used recreational drugs. Laboratory investigations revealed hypophosphatemia, reduced 1,25(OH)2D3, high serum ALP and significantly high FGF-23 levels (Table 1).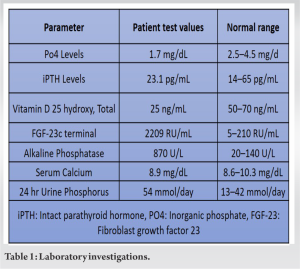

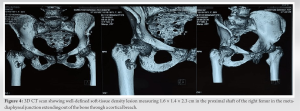
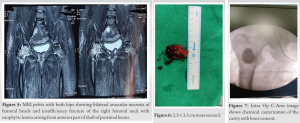
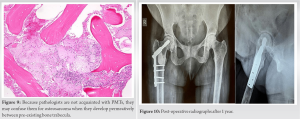
Further evaluation with a Ga68 DotA scan showed a 1.5 × 1 cm nodule in the soft tissue near the patient’s right femur. This nodule had a high concentration of 68Ga DOTATATE and a standard uptake value (max) of 4.89. The medical team suspected that the patient may have TIO due to the presence of this nodule. An additional clinicopathological correlation was required for confirmation. A whole-body FDG PET scan showed metabolic activity in the soft-tissue near the patient’s right femur. The nodule expressed SSTR, further supporting the suspicion that it was the primary lesion causing TIO. However, further clinicopathological correlation was still needed for confirmation. The patient received phosphate, calcium, and Vitamin D supplements as part of their treatment while undergoing evaluation. Based on consultation with the endocrinologist, it was decided to perform an excisional biopsy and curettage of the lesion. This procedure was done under spinal anesthesia and with the help of C-arm fluoroscopy. The orthopedic team removed a 2.5 × 3.5 cm mass from the proximal femur (Fig. 6) and chemically cauterized the area with bone cement (Fig. 7). They also conducted prophylactic fixation using DHS plating. 
Serum phosphorus levels normalized on the 7th post-operative day with a marked decreased in serum FGF 23 levels at 3-month post-operative and resulted in dramatic and remarkable clinical improvement (Table 2).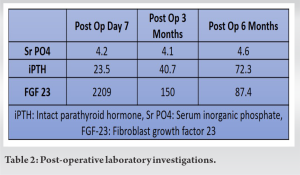
Osteomalacia is a condition characterized by inadequate or delayed mineralization of osteoid in mature bone. TIO or oncogenic osteomalacia is terms used to refer to a modest number of cases in which osteomalacia is direct as a result of the tumor. The majority of TIO cases are linked to bone tumors or mesenchymal soft-tissue tumors [5]. One particular kind of tumor is called a PMT determined to be the main cause of TIO. In 2004, a study by Folpe et al. [6] showed that the great majority of tumors associated with TIO were examples of a single histopathologic entity, now known as PMTs. In the 2013 Classification of Tumors of Soft Tissue and Bone, the World Health Organization included PMTs as a “morphologically distinctive neoplasms” that produce tumor-induced osteomalacia (TIO) in most affected patients. This is often caused by the tumor cells producing FGF23 [7]. PMTs are characterized by the production of a phosphaturic hormone or “phosphatonin” which is also called “FGF23”. Hypophosphatemia results from FGF23’s action on the kidneys, which increases phosphate excretion and inhibits phosphate reabsorption [8]. In addition, it lowers the synthesis of active Vitamin D, which lowers phosphorus absorption through the gastrointestinal system. This dysregulation of phosphate homeostasis leads to abnormal bone mineralization and development of osteomalacia [9]. Severe hypophosphatemia is the main contributory factor of TIO’s symptoms, which are unrelated to the illness itself [10]. The diagnosis of TIO can be challenging due to its non-specific symptoms, and there is often a significant delay in treatment. Patients commonly experience progressive musculoskeletal pain, muscle weakness, and bone insufficiency fractures. Misdiagnosis with other musculoskeletal or rheumatological conditions or even psychiatric conditions is common [11]. To diagnose TIO, laboratory findings typically show hypophosphatemia, hyperphosphaturia, increased levels of intact FGF23, low-normal serum calcium and PTH levels, and decreased levels of active Vitamin D. Renal tubular phosphate wasting can be confirmed through calculations of tubular reabsorption of phosphate and the TmP/GFR ratio. Various imaging modalities can be used to localize PMTs, including whole-body scans such as Tc-99m sestamibi scanning, octreotide scintigraphy, FDG-PET/computed tomography (CT), whole-body MRI, and CT. Among these, 68Ga-DOTATATE PET/CT is considered the most sensitive and specific method for detecting PMTs [12]. The primary treatment for TIO is complete surgical resection of the tumor with adequate margins. To help minimize the risk of local recurrence, it is often recommended to have a margin of 10 mm for lesions on extremities and 5 mm for lesions on the trunk [13]. A rapid resolution of symptoms and normalization of serum FGF23 and phosphate levels typically follow surgical cure. Long-term follow-up is necessary due to the possibility of late recurrence, although recurrence and metastasis are rare. For individuals who cannot have surgery or in cases where the tumor cannot be targeted, medical treatment with calcitriol and phosphate supplements may be explored. Regular follow-up with biochemical testing is essential to monitor phosphorus, calcium, alkaline phosphatase, PTH, and 24-h urine calcium levels. Adjuvant radiotherapy may be used in recurrence or incomplete resection cases, and radiofrequency ablation has been employed for inoperable tumors [14]. It is important to note that PMTs are considered benign tumors, and metastasis is uncommon, with the lung being the most common site if it does occur. Overall, early detection, accurate diagnosis, and appropriate treatment are crucial for managing TIO and improving patient outcomes.
TIO should be considered in adult patients with unexplained non-specific symptoms such as bone pain, progressive weakness, and multiple bone fractures, especially when there is no family history of similar conditions. The rarity of the condition and the non-specific nature of its symptoms often result in a delayed diagnosis. Complete resection of the tumor is the appropriate treatment of choice for TIO. The surgical intervention most often leads to the prompt and complete resolution of long-standing symptoms and biochemical abnormalities associated with TIO. In addition, it allows for the remineralization of the skeleton. Therefore, when there is suspicion of TIO, active efforts should be made to localize and resect the tumor, as this often leads to significant improvement in both clinical and biochemical parameters of the patient which was otherwise pushed to a blind end. Hypophosphatemia with elevated alkaline phosphatase levels should raise suspicion and prompt further evaluation for TIO. It is important to investigate the underlying cause of these abnormalities, including the possibility of a PMT. In conclusion, TIO must be considered part of the potential causes for patients experiencing muscle weakness and musculoskeletal pain. If hypophosphatemia with elevated alkaline phosphatase is observed, it warrants further investigation in this context. In such cases, it is crucial to identify the presence of tumors and proceed with surgical resection, as this intervention often results in significant clinical and biochemical improvement.
In patients presenting with muscle weakness and musculoskeletal pain and diagnostic dilemma exists, TIO can be included in the differential diagnosis. Timely recognition, localization, and surgical resection of the tumor are essential for achieving the resolution of symptoms and restoring normal bone mineralization.
References
- 1.Chong WH, Molinolo AA, Chen CC, Collins MT. Tumor-induced osteomalacia. Endocr Relat Cancer 2011;18:R53-77. [Google Scholar]
- 2.Jan de Beur SM. Tumor-induced osteomalacia. JAMA 2005;294:1260-7. [Google Scholar]
- 3.Beygi S, Denio A, Sharma TS. The foot that broke both hips: A case report and literature review of tumor-induced osteomalacia. Case Rep Rheumatol 2017;2017:3191673. [Google Scholar]
- 4.Higley M, Beckett B, Schmahmann S, Dacey E, Foss E. Locally aggressive and multifocal phosphaturic mesenchymal tumors: Two unusual cases of tumor-induced osteomalacia. Skeletal Radiol 2015;44:1825-31. [Google Scholar]
- 5.Boland JM, Tebben PJ, Folpe AL. Phosphaturic mesenchymal tumors: What an endocrinologist should know. J Endocrinol Invest 2018;41:1173-84. [Google Scholar]
- 6.Folpe AL, Fanburg-Smith JC, Billings SD, Bisceglia M, Bertoni F, Cho JY, et al. Most osteomalacia-associated mesenchymal tumors are a single histopathologic entity. Am J Surg Pathol 2004;28:1-30. [Google Scholar]
- 7.Mangham DC. World Health Organisation classification of tumours: Pathology and genetics of tumours of soft tissue and bone. J Bone Joint Surg Br 2004;86-B:466-6. [Google Scholar]
- 8.Riminucci M, Collins MT, Fedarko NS, Cherman N, Corsi A, White KE, et al. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest 2003;112:683-92. [Google Scholar]
- 9.Farrow EG, White KE. Tumor-induced osteomalacia. Expert Rev Endocrinol Metab 2009;4:435-42. [Google Scholar]
- 10.Piemonte S, Romagnoli E, Cipriani C, De Lucia F, Pilotto R, Diacinti D, et al. Six-year follow-up of a characteristic osteolytic lesion in a patient with tumor-induced osteomalacia. Eur J Endocrinol 2014;170:K1-4. [Google Scholar]
- 11.Leow MK, Hamijoyo L, Liew H, Thirugnanam U, Cheng MH, Loke KS, et al. Oncogenic osteomalacia presenting as a crippling illness in a young man. Lancet 2014;384:1236. [Google Scholar]
- 12.El-Maouche D, Sadowski SM, Papadakis GZ, Guthrie L, Cottle-Delisle C, Merkel R, et al. 68Ga-DOTATATE for tumor localization in tumor-induced osteomalacia. J Clin Endocrinol Metab 2016;101:3575-81. [Google Scholar]
- 13.Minisola S, Peacock M, Fukumoto S, Cipriani C, Pepe J, Tella SH, et al. Tumour-induced osteomalacia. Nat Rev Dis Primers 2017;3:17044. [Google Scholar]
- 14.Hesse E, Rosenthal H, Bastian L. Radiofrequency ablation of a tumor causing oncogenic osteomalacia. N Engl J Med 2007;357:422-4. [Google Scholar]