NGS can identify rare and difficult-to-treat organisms causing PJI.I. NGS can potentially guide therapy, culture fastidious organisms, and determine their relative abundance and confirm eradication in cases of PJI.
Dr. Henry Kuechly, Department of Orthopedic Surgery, University of Cincinnati College of Medicine, Cincinnati, Ohio. E-mail: kuechlhy@mail.uc.edu
Introduction: Prosthetic joint infections (PJIs) are a dreaded complication of joint arthroplasty. Zoonotic organisms such as Pasteurella multocida (PM) rarely cause PJIs. Still, these organisms can be challenging to treat due to a low suspicion index and inadequate growth on culture. Next-generation sequencing (NGS) can be used to identify organisms in culture-negative PJIs. This is the first reported case of a PM positive total hip arthroplasty PJI using NGS.
Case Report: We report the case of a 70-year-old male presenting with a periprosthetic hip infection. PM was identified in high relative abundance on NGS and grew in culture. Subsequent intraoperative samples were culture negative for Pasteurella, but NGS demonstrated continued presence of Pasteurella.
Conclusion: PM is a rare case of PJI, but a high index of suspicion must be maintained in the appropriate clinical context. NGS is a vital tool for the identification of culture-negative organisms like PM.
Keywords: Prosthetic joint infection, next-generation sequencing×culture negative, Pasteurella multocida, next-generation sequencing, hip infection, arthroplasty.
Prosthetic joint infection (PJI) is a dreaded complication of total joint replacement [1]. The likely causative organisms include Coagulase-negative Staphylococci, Staphylococcus aureus, Streptococci, enterococci, aerobic Gram-negative bacilli, Cutibacterium, and Enterobacterales [2-4]. Zoonotic organisms such as Pasteurella multocida (PM) very rarely cause PJIs [5]. PM is a Gram-negative coccobacillus transmitted to humans through bites or scratches from cats or dogs. Its most common manifestation includes cellulitis or osteomyelitis [6]. Typically, PJIs are confirmed using traditional gram stain, culture techniques, biomarkers (C-reactive protein [CRP], alpha-defensins, and D-dimer), and synovial fluid cell counts [7]. Unfortunately, traditional Gram stain and culture techniques are operator-dependent, time-consuming, and error-prone [8]. Furthermore, not all pathogens can grow on traditional culture methods, leading to culture-negative results with estimates ranging from 14% to 45%. Multiple bacteria may be present in the PJI microbiome, and the dominant bacteria may overwhelm the other organisms [4, 9]. Next-generation DNA sequencing (NGS) has been previously used in PJI to address the issue of culture-negative results [7]. Culture-negative PJI presents many challenges in determining prognosis, therapy, and confirming eradication. NGS has shown promise as a sensitive diagnostic tool for not only the diagnosis of bacterial infections but also the simultaneous identification of antibiotic susceptibilities [8, 10-13]. We report the case of a 70-year-old male who presented 2 months after right total hip arthroplasty (THA) for a proximal femur non-union and hardware failure with wound dehiscence and a draining sinus tract, leading to a diagnosis of PJI. Intraoperative samples were obtained for gram stain, tissue culture, and NGS, all positive for PM. Here, we report the first reported case of a PM-positive Hip PJI using NGS. The patient was informed that data concerning the case would be submitted for publication, and he provided both written and verbal consent.
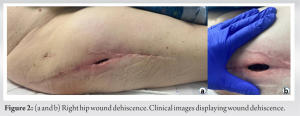
A 70-year-old male with diabetes mellitus and a surgical history notable for a right intertrochanteric femur fracture treated with a sliding hip screw complicated by a non-union and hardware failure underwent hardware removal at our hospital 18 months following the intertrochanteric fracture. Intraoperative cultures were negative at the time of hardware removal.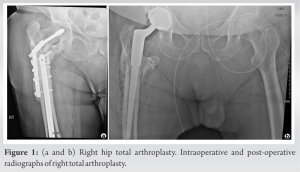
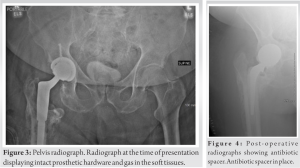
The patient reported having a domestic cat that frequently licked, playfully bit, or scratched the patient. The patient denied a history of rheumatoid arthritis, malignancy, or use of corticosteroids and other immunosuppressive agents. Laboratory workup revealed a white blood cell count of 10.4×106/uL, red blood cell count of 3.46×106/uL, and hemoglobin of 9.1 g/dL. The differential was notable for only mild elevations in basophils, absolute neutrophil counts, and absolute monocyte counts at 1.2/uL, 7862/uL, and 978/uL, respectively. Inflammatory markers were elevated, with a CRP of 293.4 mg/L and an erythrocyte sedimentation rate of 93 mm/h. A radiograph of the pelvis demonstrated intact prosthetic hardware and gas within the soft tissues (Fig. 3).
Given the diagnosis of hip PJI, the patient was taken to the operating room for irrigation and debridement, removal of THA, and placement of an antibiotic spacer (Fig. 4). Intraoperative deep tissue samples were obtained and sent for culture and NGS (Orthokey ®, Microgen, Orlando, Florida) (Fig. 5). Empiric vancomycin and 2 g intravenous cefepime Q8h were initiated postoperatively. Synovial fluid and tissue cultures were positive for PM, while anaerobic and fungal cultures were negative. NGS (Orthokey®, Microgen, Orlando, Florida) revealed a high relative abundance of PM (Fig. 5). Following these results, the patient was transitioned from empiric antibiotics to 2 g of intravenous ceftriaxone daily for 3 weeks per infectious disease recommendations. However, the above measures failed to eradicate the infection at 3 weeks, and the patient developed recurrent wound drainage, which prompted a second debridement and explantation of the spacer. A new antibiotic spacer was placed as part of a two-stage exchange with plans of returning to the OR for a THA (Fig. 6).
Three weeks following the initial irrigation and debridement with spacer placement, the patient returned to the OR for a planned reoperation with the placement of an articulating spacer and antibiotic delivery device. Intraoperative tissue samples were again obtained and sent for culture and NGS. While the deep tissue cultures were negative, NGS was positive for PM. However, the microbial burden was much less at this point this time (Fig. 7). The deep tissue samples also demonstrated >50 polymorphonuclear cells in this sample. Given the failure of the spacer and IV antibiotics, the patient received a second antibiotic-impregnated spacer and a different IV antibiotic therapy directed against PM.
The incidence of PJI is estimated to range between 0.5% and 2.3% among all joint arthroplasties [3, 14]. PM is an exceedingly rare cause of PJI and comprises just 0.1% of reported cases. Other rare potential zoonotic causes of PJI include Brucella and Coxiella burnetii [15, 16]. Roughly 35 cases of PM-associated PJI have been reported. Most of these patients report possessing a domestic cat [5, 17]. These findings are consistent with our patient, who reported regularly being licked and lightly bitten by his domestic cat. It has been speculated that patients’ knees may be more accessible than hips for domestic animals to lick or scratch, which correlates with PM PJI afflicting total knee arthroplasty 5 times more frequently than THA [5]. In addition to animal bites and scratches, previous literature has identified rheumatoid arthritis, corticosteroids, immunosuppressive therapy, and malignancy as risk factors for PM-PJI [5]. In addition to owning a domestic cat, our patient’s only risk factor for PM-associated PJI was diabetes mellitus. Although PM and other zoonotic PJIs are uncommon, as demonstrated in our case, they can be challenging to identify and treat due to either a low index of suspicion or the inability to culture the organism [15, 17] accurately. Therefore, this case illustrates the need for a high index of suspicion for these organisms in the appropriate clinical context, such as someone with multiple animals at home and comorbid conditions, leading to immune compromise. This is the first case of PM hip PJI confirmed by NGS [18]. NGS is a revolutionary technology that diagnoses clinically relevant microorganisms, quantifies their relative abundance, and detects antimicrobial resistance leading to a favorable [19]. NGS is a particularly promising tool for arthroplasty and PJI due to the well-known prevalence of culture-negative PJIs with some studies estimating culture-negative results as high as 42% [20]. Multiple previous studies have concluded that NGS can reliably identify organisms in culture-negative PJIs. This case correlates with these conclusions since the tissue obtained during the second washout was culture-negative. In contrast, NGS remained positive for PM [13, 21, 22], associated with a high neutrophil count in one of the tissue specimens obtained during his second washout. Thus, a repeat THA was deferred. Moreover, this case and the work by Tarabichi et al. illustrate that NGS is a viable option for identifying atypical organisms that may cause PJI, such as zoonotic organisms such as PM and Streptococcus canis [11]. An added benefit of NGS is the ability to more comprehensively identify multiple organisms which either may not adequately grow on culture or may grow too slowly on culture. Another potential advantage of NGS is that it detects multiple pathogens, which is beneficial in light of recent data suggesting that many PJIs are polymicrobial [12, 21].
PJI is a devastating complication of joint replacement. This complication places significant financial strain on hospital systems. More importantly, the long-term health implications for afflicted patients are substantial. For example, PJI is associated with extended hospital stays, high rates of disability, decreased quality of life, and increased hospital readmissions. Given these significant consequences, prompt identification, diagnosis, and appropriate treatment of the infected joint are paramount. NGS is a powerful and sensitive tool to accurately identify rare organism and can help select targeted antibiotic therapy.
NGS is capable of identifying rare and difficult-to-treat organisms causing PJI. NGS is potentially a vital tool for the diagnosis of PJI, especially culture-negative results, and for documenting the eradication of difficult-to-treat infections. NGS has multiple advantages over culture techniques.
References
- 1.Izakovicova P, Borens O, Trampuz A. Periprosthetic joint infection: Current concepts and outlook. EFORT Open Rev 2019;4:482-94. [Google Scholar]
- 2.Benito N, Mur I, Ribera A, Soriano A, Rodríguez-Pardo D, Sorlí L, et al. The different microbial etiology of prosthetic joint infections according to route of acquisition and time after prosthesis implantation, including the role of multidrug-resistant organisms. J Clin Med 2019;8:673. [Google Scholar]
- 3.Patel R. Periprosthetic joint infection. N Engl J Med 2023;388:251-62. [Google Scholar]
- 4.Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev 2014;27:302-45. [Google Scholar]
- 5.Honnorat E, Seng P, Savini H, Pinelli PO, Simon F, Stein A. Prosthetic joint infection caused by Pasteurella multocida: A case series and review of literature. BMC Infect Dis 2016;16:435. [Google Scholar]
- 6.Lion C, Conroy MC, Carpentier AM, Lozniewski A. Antimicrobial susceptibilities of Pasteurella strains isolated from humans. Int J Antimicrob Agents 2006;27:290-3. [Google Scholar]
- 7.Goswami K, Parvizi J, Maxwell Courtney P. Current recommendations for the diagnosis of acute and chronic PJI for hip and knee-cell counts, alpha-defensin, leukocyte esterase, next-generation sequencing. Curr Rev Musculoskelet Med 2018;11:428-38. [Google Scholar]
- 8.Chen P, Sun W, He Y. Comparison of the next-generation sequencing (NGS) technology with culture methods in the diagnosis of bacterial and fungal infections. J Thorac Dis 2020;12:4924-9. [Google Scholar]
- 9.Mizusawa M, Carroll KC. Recent updates in the development of molecular assays for the rapid identification and susceptibility testing of MRSA. Expert Rev Mol Diagn 2023;23:679-99. [Google Scholar]
- 10.Jeon YD, Lim YS, Lee S, Kim KM, Ryu CM, Jung IY, et al. A comparison between next-generation sequencing and bacterial culture for the detection of bacteria in clinical specimen. Open Forum Infect Dis 2015;2:1108. [Google Scholar]
- 11.Tarabichi M, Alvand A, Shohat N, Goswami K, Parvizi J. Diagnosis of Streptococcus canis periprosthetic joint infection: The utility of next-generation sequencing. Arthroplast Today 2018;4:20-3. [Google Scholar]
- 12.Tarabichi M, Shohat N, Goswami K, Alvand A, Silibovsky R, Belden K, et al. Diagnosis of periprosthetic joint infection: The potential of next-generation sequencing. J Bone Joint Surg Am 2018;100:147-54. [Google Scholar]
- 13.Indelli PF, Ghirardelli S, Violante B, Amanatullah DF. Next generation sequencing for pathogen detection in periprosthetic joint infections. EFORT Open Rev 2021;6:236-44. [Google Scholar]
- 14.Gundtoft PH. Prosthetic joint infection following total hip arthroplasty-incidence, mortality and validation of the diagnosis in the danish hip arthroplasty register. Dan Med J 2017;64:B5397. [Google Scholar]
- 15.Parikh MS, Antony S. A comprehensive review of the diagnosis and management of prosthetic joint infections in the absence of positive cultures. J Infect Public Health 2016;9:545-56. [Google Scholar]
- 16.Million M, Bellevegue L, Labussiere AS, Dekel M, Ferry T, Deroche P, et al. Culture-negative prosthetic joint arthritis related to Coxiella burnetii. Am J Med 2014;127:786.e7-10. [Google Scholar]
- 17.Ranavaya J, Awadh H. A case of cat bite associated Pasteurella multocida prosthetic joint infection. IDCases 2023;32:e01755. [Google Scholar]
- 18.Maritati M, Liverani L, Gigante A, Zanoli GA, De Rito G. The first case of a drug-resistant Pasteurella multocida prosthetic knee infection successfully treated with debridement, antibiotics, and implant retention. Cureus 2023;15:e38389. [Google Scholar]
- 19.Wang C, Huang Z, Li W, Fang X, Zhang W. Can metagenomic next-generation sequencing identify the pathogens responsible for culture-negative prosthetic joint infection? BMC Infect Dis 2020;20:253. [Google Scholar]
- 20.Kalbian I, Park JW, Goswami K, Lee YK, Parvizi J, Koo KH. Culture-negative periprosthetic joint infection: Prevalence, aetiology, evaluation, recommendations, and treatment. Int Orthop 2020;44:1255-61. [Google Scholar]
- 21.Goswami K, Clarkson S, Phillips CD, Dennis DA, Klatt BA, O’Malley MJ, et al. An enhanced understanding of culture-negative periprosthetic joint infection with next-generation sequencing: A multicenter study. J Bone Joint Surg Am 2022;104:1523-9. [Google Scholar]
- 22.Kullar R, Chisari E, Snyder J, Cooper C, Parvizi J, Sniffen J. Next-generation sequencing supports targeted antibiotic treatment for culture negative orthopedic infections. Clin Infect Dis 2023;76:359-64. [Google Scholar]