Clinicians should consider pyogenic arthritis in addition to reactive or viral arthritis as the differential diagnosis when acute arthritis occurs after COVID-19 infection.
Dr. Nobuhiro Kaku, Professor, Department of Orthopedic Surgery, Faculty of Medicine, Oita University, 1-1 Idaigaoka Hasama-machi, Yufu City, Oita 879-5593, Japan. E-mail: nobuhiro@oita-u.ac.jp
Introduction: COVID-19 may be associated with orthopedic symptoms, including myalgia and joint pain. There are reports of reactive arthritis and acute arthritis diagnosed after COVID-19; however, COVID-19-associated pyogenic arthritis has not been reported.
Case Report: We treated a young woman with secondary pyogenic hip arthritis that started after COVID-19. The patient was a 23-year-old woman who developed acute pain in the right hip 9 days after being diagnosed with COVID-19. Blood cultures revealed methicillin-sensitive Staphylococcus aureus and contrast-enhanced computed tomography revealed joint effusion in the right hip. Although the joint fluid culture results were negative, we suspected pyogenic arthritis of the hip joint and performed curettage and continuous irrigation of the right hip joint. Intraoperative histopathological examination of the synovial membrane revealed numerous neutrophils with segmental nuclei, consistent with a diagnosis of pyogenic arthritis.
Conclusion: To the best of our knowledge, this is the first report of probable secondary pyogenic hip arthritis in a patient with COVID-19.
Keywords: COVID-19, pyogenic arthritis, hip joint.
COVID-19 was first reported in Wuhan, China, in December 2019, and subsequently spread worldwide. The World Health Organization declared COVID-19 a pandemic in March 2020 [1]. COVID-19 was initially believed to be a respiratory disease [2]. However, its clinical course varies, with some cases being asymptomatic and others presenting a wide range of systemic symptoms in addition to respiratory tract symptoms [3-5]. COVID-19 can be associated with orthopedic diseases, including myalgia and joint pain [6]. There have also been reports of reactive arthritis and acute arthritis after COVID-19 [7-22]; however, to the best of our knowledge, no cases of COVID-19-associated pyogenic arthritis have been reported to date. We report a case of secondary pyogenic hip arthritis after COVID-19.
The patient was a 23-year-old woman who was 167.5 cm tall, weighed 62.9 kg, and had a body mass index of 22.4 kg/m2. The patient had no relevant medical history and had not been vaccinated against COVID-19. In early September 2021, she developed a fever with general malaise and tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection on polymerase chain reaction (PCR). At the time, the SARS-CoV-2 Delta (B.1.617.2) variant was prevalent in Japan. As the patient had signs of pneumonia, an intravenous infusion of 200 mg of remdesivir was administered on day 8 of COVID-19, followed by 100 mg of intravenous remdesivir once daily for the next 3 days. On day 9, the patient suddenly developed pain in the right hip, accompanied by general pain. On examination, she had tenderness in Scarpa’s triangle without redness, heat, or swelling around the hip. The range of hip motion was limited by pain on flexion (right 80°/left 120°). Blood tests on day 15 after COVID-19 onset showed an elevated C-reactive protein (CRP) level (14.63 mg/dL; reference: 0.00–0.23 mg/dL), and an elevated white blood cell (WBC) count (18.0 × 103 cells/µL; reference: 3.90 × 103–9.80×103 cells/µL) but were negative for rheumatoid factor, antinuclear antibodies, human leukocyte antigen-B27, hepatitis, and human immunodeficiency virus (HIV). Urine culture was negative; however, blood culture revealed β-lactamase-producing Staphylococcus aureus infection. An expanded low-density area was observed in the capsule of the right hip joint in the contrast-enhanced computed tomography (CT) on day 21 after the disease onset, and arthrocentesis was performed on the same day. The joint fluid was cloudy and yellowish-white in color (Fig. 1).
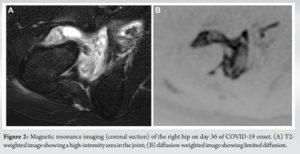
Culture of the joint fluid was negative. However, the possibility of pyogenic arthritis was considered because β lactamase-producing S. aureus had been detected on the blood culture. Therefore, cefazolin was administered intravenously on day 23 after COVID-19 onset. However, the patient’s right hip pain persisted without a change in the size of the low-density area of the right hip on CT. On day 36 after COVID-19 onset, after two negative SARS-CoV-2 PCR test results, contrast-enhanced magnetic resonance imaging (MRI) revealed restricted diffusion in the fluid reservoir of the right hip, suggesting abscess formation (Fig. 2a and b).
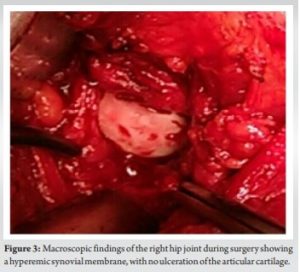
Therefore, surgical treatment with curettage and irrigation was performed on day 53, 2 weeks after the negative PCR test, according to the hospital regulations (Fig. 3).
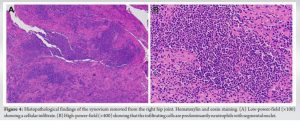
Intraoperative joint fluid culture and PCR test results for SARS-CoV-2 were negative. However, the cell count of the joint fluid was 20,800 cells/µL (neutrophil: 88%, lymphocyte: 2%, and monocyte: 10%) and the specific gravity was 1.040. Moreover, the protein and sugar levels were 8.0 g/dL and 52 mg/dL, respectively. The Synovasure a-Defensin Detection Kit (Zimmer Biomet, Warsaw, IA, USA) showed positive results, and histopathology of synovial tissue revealed numerous neutrophils with segmental lobe nuclei (Fig. 4a and b).
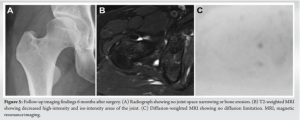
After surgery, the patient was treated with intravenous cefazolin, and the affected hip joint was continuously irrigated with 100 mg of amikacin sulfate dissolved in 1 L of saline daily for 11 days. Cultures of the drainage fluid during continuous irrigation were repeatedly negative. Oral antibiotics (1600 mg sulfamethoxazole-trimethoprim 1600/320 mg/day and rifampicin 450 mg/day) were initiated the day after intravenous antibiotic administration and continuous irrigation was completed. The patient’s right hip pain improved, and she was discharged from the hospital on day 68 after COVID-19 onset. Rifampicin was discontinued 3 months postoperatively, whereas sulfamethoxazole-trimethoprim was discontinued 4 months postoperatively. Loxoprofen was administered from day 9 after COVID-19 onset until 1 month postoperatively, but no steroids were administered. Six months after surgery, the patient had no symptoms and a Harris hip score of 96. Radiography revealed no abnormalities (Fig. 5a), and MRI showed a marked reduction in the joint effusion of the right hip. Furthermore, no diffusion restriction was observed that would suggest an abscess (Fig. 5b and c). Blood tests revealed a normal CRP level and WBC count.
Multiple cases of COVID-19-related arthritis have been reported since 2020. The patient in this report was younger than those in previous reports, and the findings, including blood culture and histopathology results, differed from those of previous reports. To date, 17 patients (10 men and seven women) with COVID-19-related arthritis have been reported (Supplementary Table S1) [7-22]. Of the 17 cases, joint fluid culture was performed in five cases, and blood culture was performed in five cases; the test results were negative in all cases. General joint fluid testing was performed in two cases, which revealed moderately elevated WBC counts (20,000 cells/µL and 18,000 cells/µL). SARS-CoV-2 PCR testing of the joint fluid was performed in four cases, and the results were negative in all four cases. Pathological examination of the synovial tissue of the joints was not performed for any of the cases. In most cases, joint symptoms occurred 2–4 weeks after the COVID-19 onset, and arthritis developed simultaneously with the COVID-19 diagnosis in only one case. The affected joints were predominantly in the legs and were monoarticular in six cases and polyarticular in seven cases, and the hands were affected in four cases. This patient was the only case of hip joint arthritis. All reported cases tested negative on bacterial culture of joint fluid and were negative for autoantibodies, rheumatoid factor, and antinuclear antibodies. Most patients were treated with steroids, non-steroidal anti-inflammatory drugs, or a combination of both for an average of 6 weeks (range, 5 days–10 months). Most patients’ symptoms improved, although two patients had persistent symptoms that did not resolve. Follow-up imaging findings of the relevant joints were reported in six cases. Viral arthritis is usually caused by parvovirus B19, hepatitis B, hepatitis C, HIV, or Epstein–Barr virus. It develops immediately after the onset of infection, presenting as symmetric polyarthritis. Symptoms are transient and often resolve spontaneously. A definitive diagnosis is made by testing the joint fluid for antibodies. However, in COVID-19-associated arthritis, the course of the disease differs from that of conventional viral arthritis, with arthritis developing more than a week after the onset of SARS-CoV-2 infection. Moreover, the causative organism could not be identified, which ruled out pyogenic arthritis, and most of the 17 cases of COVID-19-related arthritis that we reviewed were most likely reactive arthritis (ReA). ReA is generally considered a form of spondylarthritis that causes asymmetrical monoarthritis or polyarthritis following a genitourinary or gastrointestinal tract infection. There are no established criteria for the diagnosis or classification of ReA, and the 1999 workshop evaluation criteria proposed by the American College of Rheumatology [23] are often used. Eleven of the 17 reported cases of COVID-19-associated arthritis that we reviewed were clinically diagnosed as ReA. However, in six cases, the reported diagnosis was acute arthritis because the possibility of viral arthritis could not be excluded. Similarities between this case and the reported cases include an elevated WBC count, and negative PCR and joint fluid culture results. However, this case differs from previously reported cases of COVID-19-associated arthritis because β-lactamase-producing S. aureus was detected on blood culture. In addition, a histopathological examination of the synovial membrane revealed a marked proliferation of neutrophils with segmental nuclei. This finding is characteristic of pyogenic arthritis [24] and is inconsistent with that of ReA or viral arthritis, in which lymphocyte predominance is generally observed [24, 25]. Zhang et al. [26] investigated secondary infections in patients with severe COVID-19 and reported secondary infections in 22 of 38 (58%) cases. COVID-19 can cause a significant decrease in the number of peripheral lymphocytes, especially CD4 and CD8 T cells, leading to immunosuppression [27], and this may predispose patients to secondary infections.
In this case, the patient was a young woman with acute arthritis, and it was difficult to rule out a causal association with COVID-19. Hematogenous pyogenic arthritis can be directly induced in normal joints. Alternatively, bacterial arthritis can be exacerbated by concurrent non-bacterial arthritis. To the best of our knowledge, this is the first report of a patient treated for suspected secondary pyogenic arthritis within 2 weeks of onset of COVID-19. Clinicians should consider the possibility of pyogenic arthritis, in addition to ReA and viral arthritis in patients with COVID-19-associated acute arthritis, to ensure that patients are given the appropriate treatment to prevent the development of severe arthrosis.
This case suggests that COVID-19 can be associated with secondary pyogenic arthritis; therefore, in patients with COVID-19-associated acute arthritis clinicians should consider the possibility of pyogenic arthritis in the differential diagnosis, in addition to ReA and viral arthritis, to ensure that patients are given the appropriate treatment to prevent the development of severe arthrosis.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727-33. [Google Scholar]
- 2.Jiang J, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19). J Gen Intern Med 2020;35:1545-9. [Google Scholar]
- 3.Cascella M, Rajnik M, Cuomo A, Dulebohn SC, di Napoli R. Features, evaluation, and treatment of coronavirus (COVID-19). In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. [Google Scholar]
- 4.Pascarella G, Strumia A, Piliego C, Bruno F, Del Buono R, Costa F, et al. COVID-19 diagnosis and management: A comprehensive review. J Intern Med 2020;288:192-206. [Google Scholar]
- 5.Wang L, Wang Y, Ye D, Liu Q. Review of the 2019 novel coronavirus (SARS-CoV-2) based on current evidence. Int J Antimicrob Agents 2020;55:105948. [Google Scholar]
- 6.Jiang DH, Roy DJ, Gu BJ, Hassett LC, McCoy RG. Postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection: A state-of-the-art review. JACC Basic Transl Sci 2021;6:796-811. [Google Scholar]
- 7.Danssaert Z, Raum G, Hemtasilpa S. Reactive arthritis in a 37-year-old female with SARS-CoV2 infection. Cureus 2020;12:e9698. [Google Scholar]
- 8.De Stefano L, Rossi S, Montecucco C, Bugatti S. Transient monoarthritis and psoriatic skin lesions following COVID-19. Ann Rheum Dis 2023;82:e86. [Google Scholar]
- 9.Di Carlo M, Tardella M, Salaffi F. Can SARS-CoV-2 induce reactive arthritis? Clin Exp Rheumatol 2021;39:25-6. [Google Scholar]
- 10.Fragata I, Mourão AF. Coronavirus disease 19 (COVID-19) complicated with post-viral arthritis. Acta Reumatol Port 2020;45:278-80. [Google Scholar]
- 11.Gasparotto M, Framba V, Piovella C, Doria A, Iaccarino L. Post-COVID-19 arthritis: A case report and literature review. Clin Rheumatol 2021;40:3357-62. [Google Scholar]
- 12.Jali I. Reactive arthritis after COVID-19 infection. Cureus 2020;12:e11761. [Google Scholar]
- 13.Hønge BL, Hermansen ML, Storgaard M. Reactive arthritis after COVID-19. BMJ Case Rep 2021;14:e241375. [Google Scholar]
- 14.Liew IY, Mak TM, Cui L, Vasoo S, Lim XR. A case of reactive arthritis secondary to coronavirus disease 2019 infection. J Clin Rheumatol 2020;26:233. [Google Scholar]
- 15.Ono K, Kishimoto M, Shimasaki T, Uchida H, Kurai D, Deshpande GA, et al. Reactive arthritis after COVID-19 infection. RMD Open 2020;6:e001350. [Google Scholar]
- 16.Parisi S, Borrelli R, Bianchi S, Fusaro E. Viral arthritis and COVID-19. Lancet Rheumatol 2020;2:e655-7. [Google Scholar]
- 17.Saricaoglu EM, Hasanoglu I, Guner R. The first reactive arthritis case associated with COVID-19. J Med Virol 2021;93:192-3. [Google Scholar]
- 18.Talarico R, Stagnaro C, Ferro F, Carli L, Mosca M. Symmetric peripheral polyarthritis developed during SARS-CoV-2 infection. Lancet Rheumatol 2020;2:e518-9. [Google Scholar]
- 19.Yokogawa N, Minematsu N, Katano H, Suzuki T. Case of acute arthritis following SARS-CoV-2 infection. Ann Rheum Dis 2021;80:e101. [Google Scholar]
- 20.Burhan FK, Akyol A. Reactive arthritis after COVID-19: A case-based review. Rheumatol Int 2021;41:2031-9. [Google Scholar]
- 21.Cincinelli G, Di Taranto R, Orsini F, Rindone A, Murgo A, Caporali R. A case report of monoarthritis in a COVID-19 patient and literature review: Simple actions for complex times. Medicine (Baltimore) 2021;100:e26089. [Google Scholar]
- 22.Colatutto D, Sonaglia A, Zabotti A, Cereser L, Girometti R, Quartuccio L. Post-COVID-19 arthritis and sacroiliitis: Natural history with longitudinal magnetic resonance imaging study in two cases and review of the literature. Viruses 2021;13:1558. [Google Scholar]
- 23.Selmi C, Gershwin ME. Diagnosis and classification of reactive arthritis. Autoimmun Rev 2014;13:546-9. [Google Scholar]
- 24.Yanez JE, Thompson GR, Mikkelsen WM, Bartholomew LE. Rubella arthritis. Ann Intern Med 1966;64:772-7. [Google Scholar]
- 25.Armor B. Reiter’s syndrome. Diagnosis and clinical features. Rheum Dis Clin North Am 1998;24:677-95, vii. [Google Scholar]
- 26.Zhang H, Zhang Y, Wu J, Li Y, Zhou X, Li X, et al. Risks and features of secondary infections in severe and critical ill COVID-19 patients. Emerg Microbes Infect 2020;9:1958-64. [Google Scholar]
- 27.Li H, Liu L, Zhang D, Xu J, Dai H, Tang N, et al. SARS-CoV-2 and viral sepsis: Observations and hypotheses. Lancet 2020;395:1517-20. [Google Scholar]