Early detection of dysplastic changes and modulation of spinal deformities with routine follow-up surveillance X-rays is vital for early aggressive surgical planning of a dystrophic short segment kyphoscoliotic curves in NF-1.
Dr. Sai Gautham Balasubramanian, Clinical Fellow – Spinal Unit, Alderhey Children’s NHS Foundation Trust, Liverpool, L14 5AB, United Kingdom. E-mail: saigautham90@gmail.com
Introduction: Thoracic myelopathy in neuro fibromatosis-1 (NF-1) is most commonly due to intra-spinal neurofibromas/dumb-bell tumors/intra-canal rib head penetration (RHP) causing cord compression. However, acute thoracic myelopathy due to rapid progression of the kyphoscoliotic curve alone in NF-1 without a significant spinal cord compression occurs very rarely. This case report discusses our experience with one such patient and we also discuss intraoperative and post-operative challenges encountered with this patient and a rare complication of hemothorax postoperatively.
Case Report: A 15-year-old male presented to the clinic after being lost to follow-up for 4 years with a rapid acute deterioration of dystrophic curve and no myelopathic symptoms (Scoliosis – 65°, Kyphosis – 77°). His subsequent examination in 6 weeks showed acute development of myelopathic gait with right ankle and extensor hallucis longus weakness. He was admitted for halo gravity traction for 6 weeks and a single-stage posterior instrumentation with excision of rib heads at the apex was planned. Postoperatively, the patient developed massive left hemothorax and loss of power in both lower limbs at day 2. He subsequently regained full power and complete resolution of myelopathic symptoms at the end of 9-month follow-up with a satisfactory alignment of spine in the follow-up X-rays.
Conclusion: Acute onset of myelopathy is a rare and uncommon finding with a rapid deterioration of dystrophic curve alone without any major spinal cord compromise. Early detection of dysplastic changes with early aggressive surgical management and deformity correction is necessary with dystrophic NF-1 curves to prevent pre-operative and post-operative morbidities.
Keywords: Neuro fibromatosis-1, rib head penetration, myelopathy, hemothorax, halo traction.
Neuro fibromatosis Type 1 (NF-1) is an autosomal dominant hereditary disorder causing variety of peripheral nervous system and skeletal disorders. The dystrophic type of NF-1 scoliosis is manifested by dysplastic changes such as vertebral scalloping, penciling of ribs, vertebral scalloping and subluxation/dislocation, spindling of transverse process among other features [1-3]. The classic presentation of dystrophic forms is a rapid progression of an acute sharp short-segmented curve with associated hyperkyphosis, particularly in the thoracic spine [4, 5]. The principle of treatment in these dystrophic pathological acute curves is for spinal stabilization and fusion to arrest the progression of curve and maintain spinal balance coronally and sagittally. Thoracic myelopathy in NF-1 is most commonly due to intra-spinal neurofibromas/ dumb-bell tumors causing cord compression, malignant transformation of neurofibromas with intracanal hemorrhage, intra-canal rib head penetration (RHP) due to progressive curves or vertebral subluxation/dislocation into the spinal canal [6]. Neurological deficits/myelopathy are uncommon in NF-1 without the aforementioned findings due to a chronically stretched-out cord at the apex with or without dural ectasias. However, acute thoracic myelopathy due to rapid progression of the kyphoscoliotic curve alone in NF-1 without a significant spinal cord compromise/compression due to RHP/vertebral body subluxations/neurofibromas is very rare. This case report discusses our experience with a patient who was lost to follow-up for 3 years with a rapid progression of the kyphoscoliotic curve during his adolescent growth spurt and development of acute thoracic myelopathy within a period of 6 weeks from his presentation. We also discuss intra-operative and post-operative challenges encountered with this patient and also principles of treatment in such cases.
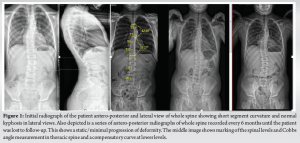
A 15-year-old male patient with a background of NF-1 and non-dystrophic scoliosis, who has been lost to follow-up for 3–4 years, presented to the clinic with acute deterioration of the curvature of his thoracic spine. He had been under the follow-up since the age of 9 years for his symptoms of NF-1 associated with a non-dystrophic curve in the thoracic spine. His initial X-rays showed a thoracic curve of around 36° with convexity to the left (T4-8; 36, Apex T6) and normal thoracic kyphosis in the lateral views (Fig. 1). He was subsequently followed-up every 6 months with a whole spine EOS standing X-rays anteroposterior (AP) and lateral views. The curve was stable with no rapid progression of scoliosis/kyphosis over the next 2 years until the patient’s 11 years of age (Fig. 1). He was then lost to follow-up for the next 3 years during the peak of COVID-19 pandemic when he was seen again in the clinic for an assessment due to a rapid acute deterioration of the dystrophic curve with back pain and a visible painful rib hump deformity developing over the past few months.
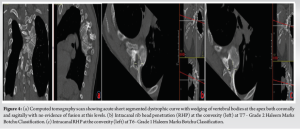
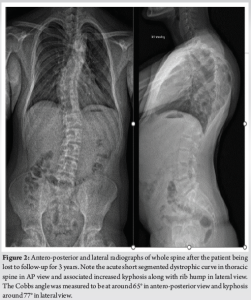
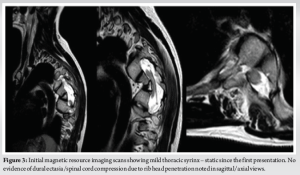
His whole spine EOS standing X-rays revealed a deterioration of his kyphoscoliotic thoracic curve with the Cobbs’ angle at 65° when measured from T4-T8. The proximal thoracic kyphosis in the X-rays was around 77° (Fig. 2). There was also modulation of the curve [7] from an earlier non-dystrophic form to a dystrophic scoliotic and hyperkyphotic thoracic curvature currently. The neurological examination was normal and bending films revealed a stiff curve with no improvement in the cobbs’ angle. The magnetic resonance (MR) scan revealed a minimal thoracic syrinx which had remained stable since his first scan 5 years ago. There were no other findings of neurofibromas/tumors/dural ectasias within his spine. The scans also showed narrowed/mildly stenotic spinal canal at the apex due to a combination of RHP and wedging of vertebral bodies without any spinal cord compression or myelomalacia (Fig. 3). computed tomography (CT) scan showed marked wedging and dysplasia of the left-sided T5/6/7 vertebral bodies due to the sharply angulated thoracic curve with apex at T6 (Fig. 4a). There was no evidence of fusion at the apex or any dysplasias in the posterior elements/rest of the spine. The CT scans also revealed Grade 2 intra-canal RHP (HMB classification) [8] without any cord impingement at T7 in the left (convexity) (Fig. 4b). This also showed rib head impingement in the foramen at left T6 level (Fig. 4c) (Grade 1).
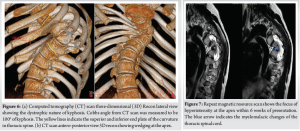
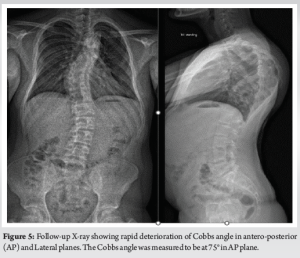
He was seen again in the clinic after the scans in 6-week time for consenting and admission as he was complaining of myelopathic symptoms for the past few days. On clinical examination, he was found to have myelopathic gait, sustained clonus, and all the upper motor neuron (UMN) signs in his lower limbs were positive. The X-ray revealed further deterioration of his kyphoscoliotic curve. The Cobbs angle was around 75° T4-8, apex at T6/7, with a compensatory flexible curve in TL spine of around 35° in his AP views and the kyphosis was measured to be at around 100° in his proximal thoracic spine (Fig. 5, 6a and b). He was referred again for urgent MR and CT scans as he had developed an acute myelopathy with deterioration of kyphoscoliosis of thoracic spine. The MR scan revealed a focus of hyper-intense signal changes in the cord at the apex T6/7 (Fig. 7). The spinal cord was draped around the vertebral bodies posteriorly at the convexity of the curve along with aforementioned CT scan findings of RHP into the canal without any undue spinal cord compression.
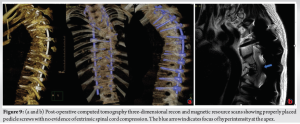
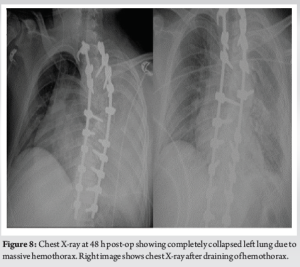
The patient was subsequently admitted for halo gravity traction (HGT) for 6 weeks and the baseline neurology was recorded. His motor examination revealed right-sided ankle Dorsiflexion and extensor hallucis longus weakness (Medical Research Council [MRC] 3/5). The rest of the neurology was normal. The UMN signs were positive in his bilateral lower limbs with spasticity more marked on his left side. There was a minimal improvement of his kyphosis with 6 weeks of lying and sitting traction with traction X-rays taken at 3rd and 6th week. His neurology was unchanged at the end of 6 weeks. A single-stage posterior instrumentation and deformity correction with removal of left T6 and T7 rib heads was planned with neuro-monitoring. The supine and prone SSEP and MEP baselines were recorded and T3-T12 pedicle screws with bilateral T2 hooks were inserted. The proximal metalworks were implanted with O-Arm navigation guidance. All the metalworks were checked intra-operatively with two surgeon check of pedicle screw pilot holes and with an intra-op CT scan and fluroscopy. The intraoperative neuromonitoring was unchanged from baseline throughout the procedure. Before the correction of kyphoscoliotic deformity, both rib heads on the left T6 and T7 were resected from the canal and foramen, respectively (5 cm of rib resected approx.), and full Ponte’s osteotomy was done at T6/7 level. Partial curve correction was achieved with direct vertebral rotation at the concave apex with cantilever techniques at the apical convex side to reduce rib hump and excess kyphosis at the proximal thoracic spine. Copious amounts of morselized autologous bone graft were placed posteriorly to promote posterior bony fusion. Time for surgery was 6 h and blood loss was 750 mL which was harvested from cell saver and transfused again. There were no intraoperative adverse events and care was taken for complete meticulous hemostasis after rib head resection. The post-operative period was uneventful for the initial 24 h and the patient was transferred back toward safely with a wound drain in situ. On post-operative day 2, his saturation dropped to 92% with symptoms of dyspnea and tachycardia. His left-sided breath sounds were decreased with dullness to percussion. The subsequent chest X-ray showed pneumohemothorax which had accumulated over the period of 48 h and had produced a massive hemothorax episode acutely (Fig. 8). This was drained as an emergency in the wards with insertion of chest drain. The chest drain collected around 1.2 L blood within an hour and a further 500 ml over the next 24 h. He was transferred to high dependency unit for intensive monitoring and subsequent chest X-rays were clear (Fig. 9). However, after draining the hemothorax his neurology dropped to 1–2/5 MRC grading in both his lower limbs in all groups of muscles. The subsequent CT scan (Fig. 9a, b) performed did not show any evidence of implant malposition or epidural hematoma compressing the spinal cord which might explain the sudden rapid onset of neurodeficits. MR scan of his whole spine was also performed which showed residual focus of hyper-intense signal changes at T6/7 with no evidence of residual spinal cord compression.
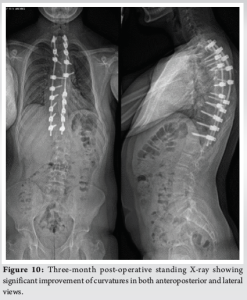
His presentation was discussed in multi-disciplinary mortality and morbidity meeting where it was thought to be an isolated acute hypotensive ischemic insult to the spinal cord. He was placed on bed rest and was started on a course of 4 mg dexamethasone three times a day for 7 days. The patient subsequently recovered over next 2 weeks with neurology back to baseline as pre-op. The patient had a long intense rehabilitative course over the next 2 months and his neurology at the time of discharge was 5/5 MRC grading with reduction of UMN signs in his lower limbs. The patient progressed through various stages with the physiotherapists and ultimately discharged on walking with the help of walking stick. His check X-rays at 6-month (Fig. 10) post-operative period showed satisfactory metalwork in situ. The kyphosis in his proximal thoracic spine was reduced and overall sagittal and coronal balance of the spine was satisfactory. The subsequent post-operative follow-up at 9 months showed almost complete resolution of myelopathic gait and dampening of UMN signs in lower limbs.
Dystrophic curves have a strong predisposition to deteriorate further and should be treated aggressively with surgical intervention as early as possible even in skeletally immature patients with Cobbs angle >40 [9, 10]. The type of fusion posterior or anterior or both usually depends on the magnitude of the curve and skeletal maturity at presentation. Posterior only fusion is usually recommended and can be used successfully for correction of dystrophic curves with kyphosis <95° [10] and scoliosis <90° using a long segment instrumented fusion including both stable and neutral vertebrae and abundant bone grafts [11]. A much extensive osteotomy and deformity correction strategies such as pedicle subtraction osteotomy/vertebral column resection can also be successfully avoided in the presence of mobile discs at the apex without any fusion/subluxation of vertebral bodies. Anterior approaches can be highly challenging and risky in hyperkyphosis due to severe crowding of vital organs and excess vascularity with plexiform venous channels in NF-1 [12, 13]. Furthermore, anterior strut grafts in the convexity can be technically challenging due to severe dysplastic changes and wedging of the vertebral bodies, thus providing insufficient mechanical anterior support [14]. Pre-operative HGT is usually indicated in severe rigid curves, especially in cases associated with sagittal plane deformity and neurological deficits. Koller et al. [15] in his review of the literature found that pre-operative halo traction might be particularly beneficial in cases with severe decompensating kyphoscoliosis and evolving neurological deficits with spinal cord draped around the apex of the curve. The emphasis on HGT after anterior releases for major deformity correction is well documented in the literature [16]. More rigid and fixed kyphoscoliotic curves are less amenable to correction with just HGT without prior procedure. The main purpose of HGT in decompensating rigid curves is to relieve the tension in the apex, thus allowing relaxation of spinal cord even if the correction of deformity is minimal. Intra-canal rib head dislocation associated with spinal canal stenosis and neurological deficits in dystrophic short segment curves in NF-1 has been widely reported in the literature [17-23]. Correction of such deformities without rib head resection might result in catastrophic RHP into the spinal cord and cause neurological deficits [24-26]. Rib head excision at the apical convexity is generally advocated as it is a simple procedure, provides copious bone grafts, provides cosmetic relief of rib humps, and also avoids the peri-operative complications of spinal cord injury. The associated decline in pulmonary functions is only minimal and short-lasting during the initial post-op period. The overall incidence of pulmonary complications is 0.9% and hemothorax is especially rare with incidence of <0.1% in literature in scoliosis correction surgeries with cystoplasty [27]. Painful rib hump was reported as a new clinical indicator for rib head subluxation due to mobile rib head by Gkiokas et al. [28]. RHP is classified into three grades from CT scans by Haleem-Marks-Botchu classification [8] based on the location and extent of rib head protrusion into spinal canal. Grade 3 has a high relative risk of spinal cord compression and neurological deficits, while Grades 1 and 2 are classified as low and medium risk. Durrani et al. [7] reported that non-dystrophic curves can progress to dystrophic ones by acquiring new dysplastic features which might not be present during the initial X-ray. Durrani et al. described these changes as “modulation” which is unique to spinal deformities associated with NF-1. However, the presence of modulation in follow-up X-rays did not necessarily mean the progression of curves or vice-versa. A careful, meticulous evidence of dysplastic features in X-rays is essential in final prognosis and further planning of management strategies. Thoracic myelopathy due to rapid progression of kyphoscoliotic curve in the absence of cord impingement due to rib head dislocation/neurofibromas is very rare and was never reported in the literature in neurofibromatosis. In our case report, the patient presented with a severe short dystrophic kyphoscoliotic curve at his initial presentation, after being lost to follow-up with a non-dystrophic curve, with intra-canal RHP of Grade 2 HMB RHP at left T7 and a painful rib hump. He subsequently developed myelopathy and neurological deficit within 6 weeks with worsening of kyphoscoliotic curve. The authors believe that worsening of kyphosis with draping of spinal cord over the concavity of the apex might be the reason for sudden onset of neurological deficits without an obvious cause of spinal cord compression apart from mild RHP at the apex. This case report also highlights the importance of compulsory regular follow-ups every 6 months to record the X-rays and the clinical status as NF-1 can lead to a rapid deterioration of the kyphoscoliotic curve with adolescent growth spurts. The signs of dysplasia associated with the NF-1 curve should be carefully evaluated and documented as the prognosis and management strategy drastically changes with the diagnosis of dystrophic curves. The follow-up X-rays should also be carefully evaluated for modulation of bony anatomy with the development of new dystrophic features in a previously non-dystophic curve.
We presented a case report of NF-1 with acute onset of myelopathy mainly due to rapid progression of kyphoscoliosis in a dystrophic curve in the absence of any major spinal cord compression from RHP. Early detection of dysplastic changes and modulation of spinal deformities with routine follow-up surveillance X-rays is vital in further management planning in dystrophic short segment kyphoscoliotic curves in NF-1. Early aggressive surgical management and posterior instrumented deformity correction are necessary to prevent post-operative morbidities.
Dystrophic curves in NF-1 is associated with a rapid progression of kyphoscoliotic deformities with adverse clinical outcomes. The spinal modulation from a non-dystrophic to dystrophic pattern of the curve should be carefully evaluated in follow-up X-rays to predict the prognosis and management strategies. Thoracic myelopathy due to the rapid progression of kyphoscoliotic curve alone in the absence of cord impingement is very rare in NF-1. Early detection of dysplastic changes and modulation of spinal deformities with routine follow-up surveillance X-rays is vital for early aggressive surgical planning of a dystrophic short segment kyphoscoliotic curves in NF-1.
References
- 1.Akbarnia BA, Gabriel KR, Beckman E, Chalk D. Prevalence of scoliosis in neurofibromatosis. Spine (Phila Pa 1976) 1992;17:S244-8. [Google Scholar]
- 2.Crawford AH, Parikh S, Schorry EK, Von Stein D. The immature spine in type-1 neurofibromatosis. J Bone Joint Surg Am 2007;89:123-42. [Google Scholar]
- 3.Vitale MG, Guha A, Skaggs DL. Orthopaedic manifestations of neurofibromatosis in children: An update. Clin Orthop Relat Res 2002;401:107-18. [Google Scholar]
- 4.Winter RB, Moe JH, Bradford DS, Lonstein JE, Pedras CV, Weber AH. Spine deformity in neurofibromatosis. A review of one hundred and two patients. J Bone Joint Surg Am 1979;61:677-94. [Google Scholar]
- 5.Funasaki H, Winter RB, Lonstein JB, Denis F. Pathophysiology of spinal deformities in neurofibromatosis. An analysis of seventy-one patients who had curves associated with dystrophic changes. J Bone Joint Surg Am 1994;76:692-700. [Google Scholar]
- 6.Crawford AH Jr., Bagamery N. Osseous manifestations of neurofibromatosis in childhood. J Pediatr Orthop 1986;6:72-88. [Google Scholar]
- 7.Durrani AA, Crawford AH, Chouhdry SN, Saifuddin A, Morley TR. Modulation of spinal deformities in patients with neurofibromatosis type 1. Spine (Phila Pa 1976) 2000;25:69-75. [Google Scholar]
- 8.Haleem S, Malik M, Azzopardi C, Botchu R, Marks DS. The haleem-marks-botchu classification: A novel CT based classification for intracanal rib head penetration. Spine Deform 2021;9:1651-7. [Google Scholar]
- 9.Sponseller P. The spine in skeletal syndromes and dysplasias. In: DeWald RL, editor. Spinal Deformities: The Comprehensive Text. New York: Thieme; 2003. p. 701-71. [Google Scholar]
- 10.Shen JX, Qiu GX, Wang YP, Zhao Y, Ye QB, Wu ZK. Surgical treatment of scoliosis caused by neurofibromatosis type 1. Chin Med Sci J 2005;20:88-92. [Google Scholar]
- 11.Li M, Fang X, Li Y, Ni J, Gu S, Zhu X. Successful use of posterior instrumented spinal fusion alone for scoliosis in 19 patients with neurofibromatosis type-1 followed up for at least 25 months. Arch Orthop Trauma Surg 2009;129:915-21. [Google Scholar]
- 12.Crawford AH. Pitfalls of spinal deformities associated with neurofibromatosis in children. Clin Orthop Relat Res1989;245:29-42. [Google Scholar]
- 13.Crawford AH, Herrera-Soto J. Scoliosis associated with neurofibromatosis. Orthop Clin North Am 2007;38:553-62, vii. [Google Scholar]
- 14.Shah MS, Akbary K, Patel PM, Nene AM. Management of proximal thoracic kyphoscoliosis with early myelopathy in a young adult with neurofibromatosis type 1: A case report and review of literature. J Orthop Case Rep 2020;10:8-12. [Google Scholar]
- 15.Koller H, Zenner J, Gajic V, Meier O, Ferraris L, Hitzl W. The impact of halo-gravity traction on curve rigidity and pulmonary function in the treatment of severe and rigid scoliosis and kyphoscoliosis: A clinical study and narrative review of the literature. Eur Spine J 2012;21:514-29. [Google Scholar]
- 16.Chung WH, Mihara Y, Toyat SS, Chiu CK, Hasan MS, Saw A, et al. Pre-operative halo-pelvic traction for neurofibromatosis patients with severe proximal thoracic spinal deformity: Indications and early treatment outcome. Malays Orthop J 2021;15:99-107. [Google Scholar]
- 17.Gao R, Guo D, Zhang X, Sun B, Yao Z, Cao J, et al. Surgical treatment of the intraspinal rib head dislocation in children with dystrophic scoliosis secondary to type 1 neurofibromatosis. J Pediatr Orthop 2022;42:e242-9. [Google Scholar]
- 18.Chong KL, Lam KS, Zuki Z. Dystrophic scoliosis in neurofibromatosis and rib-head resection: A case report. Malays Orthop J 2017;11:59-62. [Google Scholar]
- 19.Yalcin N, Bar-on E, Yazici M. Impingement of spinal cord by dislocated rib in dystrophic scoliosis secondary to neurofibromatosis type 1: Radiological signs and management strategies. Spine (Phila Pa 1976) 2008;33:E881-6. [Google Scholar]
- 20.Dacher NJ, Zakine S, Monroc M, Eurin D, Lechevallier J, Le Dosseur P. Rib displacement threatening the spinal cord in a scoliotic child with neurofibromatosis. Pediatr Radiol 1995;25:58-9. [Google Scholar]
- 21.Deguchi M, Kawakami N, Saito H, Arao K, Mimatsu K, Iwata H. Paraparesis after rib penetration of the spinal canal in neurofibromatous scoliosis. J Spinal Disord 1995;8:363-7. [Google Scholar]
- 22.Kamath VS, Kleinman KP, Ragland RL, Tenreiro-Picon OR, Knorr JR, Davidson RI, et al. Intraspinal dislocation of the rib in neurofibromatosis: A case report. Pediatr Radiol 1995;25:538-9. [Google Scholar]
- 23.Major RM, Huizenga AB. Spinal cord compression by displaced ribs in neurofibromatosis. A report of three cases. J Bone Joint Surg Am 1988;70:1100-2. [Google Scholar]
- 24.Khoshhal KI, Ellis RD. Paraparesis after posterior spinal fusion in neurofibromatosis secondary to rib displacement: Case report and literature review. J Pediatr Orthop 2000;20:799-801. [Google Scholar]
- 25.Mao S, Shi B, Wang S, Zhu C, Zhu Z, Qian B, et al. Migration of the penetrated rib head following deformity correction surgery without rib head excision in dystrophic scoliosis secondary to type 1 neurofibromatosis. Eur Spine J 2015;24:1502-9. [Google Scholar]
- 26.Capella M, Bettini N, Dema E, Girardo M, Cervellati S. Late post-operative paraparesis after rib penetration of the spinal canal in a patient with neurofibromatous scoliosis. J Orthop Traumatol 2008;9:163-6. [Google Scholar]
- 27.Fu KM, Smith JS, Polly DW, Ames CP, Berven SH, Perra JH, et al. Morbidity and mortality associated with spinal surgery in children: A review of the scoliosis research society morbidity and mortality database. J Neurosurg Pediatr 2011;7:37-41. [Google Scholar]
- 28.Gkiokas A, Hadzimichalis S, Vasiliadis E, Katsalouli M, Kannas G. Painful rib hump: A new clinical sign for detecting intraspinal rib displacement in scoliosis due to neurofibromatosis. Scoliosis 2006;1:10. [Google Scholar]