Core decompression with bone marrow aspirate concentrate (BMAC) implantation demonstrates significant potential in delaying the need for more invasive treatments and improving pain and functional outcomes in osteonecrosis of the femoral head (ONFH).
Dr. Naveen Jeyaraman, Department of Orthopaedics, ACS Medical College and Hospital, Dr. MGR Educational and Research Institute, Chennai, Tamil Nadu, India. E-mail: naveenjeyaraman@yahoo.com
Introduction: Osteonecrosis of the femoral head (ONFH), resulting from impaired blood supply to the head of the femur, presents a significant challenge to clinicians due to its debilitating nature. Conservative treatment often offers insufficient pain relief and debilitating functional outcomes which necessitate alternative therapies. Bone marrow aspirate concentrate (BMAC), a potent orthobiologics and rich in mesenchymal stromal cells and growth factors, holds good promise as the minimally invasive procedure for ONFH. With the preceding research suggesting clinical and functional efficacy, we assessed the therapeutic effectiveness of BMAC in ONFH management in joint preservation.
Materials and Methods: A prospective cohort study was conducted with 20 patients suffering from ONFH who failed to respond to 6 months of conservative treatment. A uniform surgical procedure was performed by a single surgeon, involving bone marrow extraction from the anterior iliac crest and subsequent processing into an 8–10 mL of BMAC concentrate. The BMAC was then injected into the implanted into the decompressed femoral head. The post-operative protocol comprised weight-bearing mobilization, physiotherapy, and a 4-week NSAID-free regimen. Outcome measures included pain scores, hip function, knee symptoms, sports activities, patient satisfaction, and recommendation of the procedure.
Results: Of the 20 patients suffering from ONFH, primarily the left side, most of whom were at stage 2b, significant pain reduction and functional improvement were observed over 24 months. The mean pain score decreased from 9.00 to 3.55, while the hip function score increased from 46.12 to 88.60. However, some patients encountered complications such as symptom recurrence (5%), disease progression (10%), and persistent pain (5%).
Conclusion: Core decompression with BMAC implantation emerges as a promising, effective, and safe treatment for ONFH with better cost-effectiveness and minimal side effects, making it a feasible treatment alternative.
Keywords: Femoral head, osteonecrosis, bone marrow aspirate concentrate, decompression.
Osteonecrosis of the femoral head (ONFH), a variant of osteonecrosis, arises due to an interruption in the blood supply of the proximal femur [1]. This subtype of osteonecrosis manifests following a disruption of the vascularization to the proximal femur. The incidence rate of ONFH observed through new case registrations per annum varies from 10,000 to 20,000 depending on geographical location [2]. Etiologies of the ONFH condition are multifactorial, encompassing both traumatic (fractures and dislocations) and non-traumatic (chronic alcohol use, long-term steroid consumption, coagulopathies, and congenital) causes [3-5]. The debilitating impact of ONFH necessitates early vigilance by clinicians to detect early signs of disease manifestation [6]. An exception to racial disparities in the incidence of ONFH is observed in sickle cell disease, which demonstrates a higher prevalence among individuals of African descent [7,8]. Gender differences in the likelihood of disease occurrence are observed, with males presenting a three to five-fold higher risk compared to females [9,10]. Bone marrow aspirate concentrate (BMAC) presents as a potential therapeutic solution for ONFH, functioning as an autologous product that undergoes minimal manipulation following its derivation from bone marrow aspiration [11,12]. As a heterogeneous cellular composition, BMAC encompasses mesenchymal stem cells (MSCs), hematopoietic stem cells, endothelial progenitor cells, and platelets, while simultaneously encompassing a variety of growth factors and cytokines [13]. The therapeutic potential of BMAC is underscored by its various bioactive properties that have been observed in vitro and animal models, such as anti-inflammatory, immunomodulatory, angiogenic, and chondrogenic properties [14,15]. Importantly, these properties are instrumental in the pathophysiological processes of osteonecrosis, therefore positioning BMAC as a promising approach in the management of this condition. An additional advantage is the relative ease of obtaining and processing BMAC at the point of care. This convenience is facilitated by the availability of commercial devices specifically designed for BMAC collection and processing, thereby further underpinning the potential role of BMAC in the clinical setting [16,17]. The previous literature has shown that the use of BMAC, in osteonecrosis, provides better results concerning pain and functional outcomes [18-21]. This study aims to critically evaluate the therapeutic effectiveness and functional outcome of BMAC implantation in the management of ONFH.
After obtaining Institutional Ethics Committee approval and written informed consent from all the participants, a prospective cohort study was conducted between March 2022 and February 2024. A total of 20 patients with radiographic evidence of idiopathic ONFH (stage 1, 2a, or 2b according to Modified Ficat and Arlet classification) who showed no symptomatic relief from the conservative line of management for 6 months were included in the study. Patients with other causes of ONFH and patients with Stage 3 or 4 ONFH according to Modified Ficat and Arlet classification [22] were excluded from the study.
All patients who met the inclusion criteria were counseled for the procedure and informed consent was obtained. All the procedures were performed by a single surgeon. Before the start of surgery, the 13G aspiration needle (Bone Access Needle (Medtronic, Inc) was flushed with 5000 U heparin mixed with 20 mL of distilled water, and then six 10 mL syringes were filled with 1 mL of diluted heparin solution.
The procedure was carried out under spinal anesthesia for all patients. Parts were prepared and draped maintaining all aseptic precautions. Bone marrow was aspirated from the ipsilateral anterior iliac crest. After performing a small 2 cm incision behind the iliac crest, a 13G needle was introduced using the sharp stylet. Once, the medullary space was accessed, the sharp stylet was exchanged for the blunt stylet with the access needle being advanced to the desired depth. The aspiration cannula was then connected to a 10 mL syringe and bone marrow was aspirated with manipulation in different directions to allow access to untapped bone marrow. A total of 60 mL of bone marrow was harvested for a single hip.
Following extraction, the aspirate was transferred to sterile tubes which were then processed in a separate room under sterile conditions to isolate the buffy coat through centrifugation. A double spin technique was used for centrifugation (Rotofix 32A centrifuge, 15 min at 2800 rpm followed by 15 min at 3600 rpm) which yielded around 8–10 mL of concentrate for every 60 mL of BMAC.
The BMAC was then transported back to the operating room maintaining utmost sterility. The core decompression (CD) was performed in the involved hip with 4 mm drill bit for three cores (to remove the necrotic core) and then injected with the BMAC concentrate (at the rate of 2 million BMAC cells per kilogram body weight divided for two hips) using an 18G spinal and sealed with bone wax. The hip joints were passively moved throughout its range of motion immediately after the injection to disperse the fluid throughout the joint.
Post-operative protocol
Patients were started on full-weight-bearing mobilization immediately post-surgery. Physiotherapy included quadriceps and hamstring strengthening exercises and patients were permitted to do light activities as tolerated. After 6–8 weeks, patients were permitted to resume full activity.
Outcome measures
All patients had regular follow-ups (pre-procedure and at the end of 1, 3, 6, 12, and 24 months) with scores being evaluated in person through direct examination. Patients were evaluated using a VAS pain score (0–10, with 10 being the most severe); and modified Harris hip score (MHHS) [23] is a self-administered tool ranging from 0 to 100, with 100 representing the highest possible level of function and lowest degree of symptoms.
Statistical analysis
Statistical analysis was performed with SPSS software version 26.0, IBM Corp, Chicago, Illinois, USA. Data normality was evaluated using the Shapiro–Wilk test. Continuous variables were presented as means with standard deviation. Comparison of VAS and MHH score parameters at subsequent follow-ups was analyzed using the paired samples t-test. Data are presented as histograms and P < 0.05 was considered significant with a 95% confidence interval.
The study comprised participants with an average age of 43.65 ± 13.62 years, ranging from a minimum of 25 to a maximum of 64 years. A majority of participants (65%) presented with the left hip involvement, while 35% had a right hip affliction. According to the modified Ficat and Arlet staging, the distribution was as follows: 30% in Stage 1, 15% in Stage 2a, and 55% in Stage 2b. All 20 cases were identified as having idiopathic etiology.
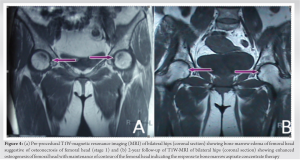
The mean visual analog scale (VAS) scores demonstrated a significant reduction over the study period. The scores decreased from a pre-operative mean of 9.00 ± 0.00 to 3.55 ± 0.51 at the 24-month follow-up. Correspondingly, the mean (± SD) MHHS showed substantial improvement in terms of pain, function (limp, support, and distance walked), functional activities (stairs, squatting, sitting cross-legged, and public transportation), and hip range of motion, increasing from 46.12 ± 3.68 preoperatively to 88.60 ± 5.06 at 24 months. The timeline distribution of these scores is presented in Table 1, Fig. 1 and 2. The statistical analysis revealed a significant change in both VAS and MHHS scores over time (P < 0.001 for both). Furthermore, we calculated the percentage change in scores at each time point relative to the pre-operative measurements, as shown in Table 2. This approach provides a clearer depiction of the progression over time. Regarding complications, we observed the following: Recurrence of symptoms in one patient (5%), progression of disease in two patients (10%), and persistent hip pain in one patient (5%). A follow-up radiograph and MRI of a representative case are depicted in Fig. 3 and 4.
ONFH is a complex condition with significant morbidity, where orthobiologics play a major role in the management protocol. BMAC, a potential orthobiologics, contains a high concentration of MSCs, growth factors, and cytokines that accelerate the body’s natural healing and tissue regeneration process. BMAC implantation is minimally invasive, low-risk, and cost-effective. It has demonstrated success in reducing pain, increasing patient activity, and improving overall outcomes. Consequently, BMAC implantation is a viable therapeutic option for ONFH patients, offering the potential to enhance their quality of life. A hypothesis suggests that ONFH may result from an insufficient supply of progenitor cells in the femoral head and proximal femur, hindering tissue remodeling. To address this issue, bone grafting combined with stem cell infiltration is an attractive treatment approach. This method not only decompresses the femoral head but also introduces osteoconductive and osteoinductive materials. Importantly, it preserves the natural hip joint geometry and articular cartilage, contributing to hip joint preservation. In addition, newer treatments focus on repairing the femoral head architecture and involve the introduction of stem cells to necrotic sites, addressing potential progenitor cell deficiencies. The intricate realm of orthopedic research, particularly in addressing ONFH, reveals a dynamic and complex treatment landscape. Our study, delving into the efficacy of CD combined with autologous BMAC, provides insights that are both corroborative and distinctive when juxtaposed with existing literature. The nuanced outcomes observed in our cohort of 40 femoral heads in 20 patients, with a notable radiographic progression-free survival (PFS) and conversion to total hip arthroplasty (THA) colostomy-free survival (CFS) rates, echo a broader narrative in ONFH management. Our findings, showing a PFS of 78.3% at 2 years and 53.3% at 5 years, alongside a CFS of 72.1% and 54.6%, respectively, at these intervals, resonate with the overarching objective of delaying disease progression and reducing the necessity for THA. These outcomes, when evaluated against a spectrum of studies, indicate a consistent trend toward the effectiveness of CD and BMAC in early to mid-stage ONFH. For instance, a 10-year retrospective study encompassing 69 patients (109 hips) demonstrated the long-term viability of concentrated autologous bone marrow aspirate transplantation, with an overall THA conversion rate of 34% [24]. This longitudinal perspective is crucial as it provides a comprehensive view of the intervention’s durability and efficacy over time. Our study’s demographic profile, predominantly idiopathic in etiology with an average participant age of 43.65 years, is reflective of the broader demographic trends observed in ONFH research. This consistency across studies is pivotal in understanding disease etiology, progression, and response to treatment. Our observed improvements in pain and functional scores, particularly the significant reductions in VAS scores and enhancements in MHHS scores, are in line with similar improvements reported in other studies, although with variations in scales and scoring systems [24-27]. The treatment’s efficacy, however, must be considered in light of its limitations. The diversity in patient demographics, disease staging, and treatment modalities across studies, including ours, poses challenges in deriving generalized conclusions. For example, a randomized controlled trial comparing CD plus saline injection against CD plus BMAC implantation in stage three non-traumatic osteonecrosis reported no significant improvement in disease progression, thereby indicating limited efficacy in advanced disease stages [28]. This finding is critical as it underscores the nuanced relationship between disease stage and treatment effectiveness, emphasizing the necessity of early intervention. Moreover, our study, along with comparative research, places significant emphasis on complication rates and disease progression as key metrics of treatment success. While our study reported a comparatively lower complication rate, this could be reflective of either the treatment’s efficacy or the predominantly early stage of disease in our participants. Other studies report varied frequencies and types of complications, underlining the importance of a comprehensive approach to post-treatment monitoring and patient care [28,29]. The statistical significance of changes in VAS and MHHS scores in our study, which is consistent with the positive outcomes reported in the literature, reinforces the potential of targeted interventions in managing ONFH effectively [24-27]. However, the need for more standardized outcome measures and reporting formats across studies is clear. Such standardization would facilitate more direct comparisons, enabling a deeper understanding of treatment impacts and broader implications. The limitations of this study encompass a relatively small sample size of 40 femoral heads in 20 patients, primarily with idiopathic ONFH, potentially limiting its generalizability to various ONFH etiologies. The retrospective design and absence of a control group hinder the establishment of causal relationships. The 2-year follow-up mainly captures short-to-medium-term outcomes, leaving long-term efficacy uncertain. Variability in disease stages among patients may have influenced treatment outcomes, particularly in advanced ONFH stages. Relying on subjective outcome measures such as VAS and MHHS scores introduces potential reporting biases, suggesting the need for objective assessments. The study also lacks quantification of progenitor cells in bone marrow concentrate, a significant factor for BMAC therapy success. Furthermore, factors such as lifestyle, comorbidities, and medication use were not comprehensively controlled for or analyzed, potentially influencing outcomes. The variability in CD and BMAC techniques across studies underscores the importance of standardized protocols for result comparability. Ethical considerations and the specialized nature of bone marrow aspiration may limit the widespread application of the findings from this study.
CD with BMAC implantation offers a promising, minimally invasive treatment, potentially delaying the need for interventions like THA in ONFH, as evidenced by reduced pain (VAS scores) and improved functional outcomes (MHHS) over 24 months.
CD combined with BMAC implantation offers a promising treatment for osteonecrosis of the femoral head, significantly improving pain and functional outcomes and potentially reducing the need for total hip arthroplasty.
References
- 1.Petek D, Hannouche D, Suva D. Osteonecrosis of the femoral head: Pathophysiology and current concepts of treatment. EFORT Open Rev 2019;4:85-97. [Google Scholar]
- 2.Moya-Angeler J, Gianakos AL, Villa JC, Ni A, Lane JM. Current concepts on osteonecrosis of the femoral head. World J Orthop 2015;6:590-601. [Google Scholar]
- 3.Rezus E, Tamba BI, Badescu MC, Popescu D, Bratoiu I, Rezus C. Osteonecrosis of the femoral head in patients with hypercoagulability-from pathophysiology to therapeutic implications. Int J Mol Sci 2021;22:6801. [Google Scholar]
- 4.Baig SA, Baig M. Osteonecrosis of the femoral head: Etiology, investigations, and management. Cureus 2018;10:e3171. [Google Scholar]
- 5.Barney J, Piuzzi NS, Akhondi H. Femoral head avascular necrosis. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2023. Available from: http://www.ncbi.nlm.nih.gov/books/NBK546658 [Last accessed on 2023 Aug 22]. [Google Scholar]
- 6.Ikeuchi K, Hasegawa Y, Seki T, Takegami Y, Amano T, Ishiguro N. Epidemiology of nontraumatic osteonecrosis of the femoral head in Japan. Mod Rheumatol 2015;25:278-81. [Google Scholar]
- 7.Adesina O, Brunson A, Keegan TH, Wun T. Osteonecrosis of the femoral head in sickle cell disease: Prevalence, comorbidities, and surgical outcomes in California. Blood Adv 2017;1:1287-95. [Google Scholar]
- 8.Adesina OO, Neumayr LD. Osteonecrosis in sickle cell disease: An update on risk factors, diagnosis, and management. Hematology Am Soc Hematol Educ Program 2019;2019:351-8. [Google Scholar]
- 9.Vardhan H, Tripathy SK, Sen RK, Aggarwal S, Goyal T. Epidemiological profile of femoral head osteonecrosis in the North Indian population. Indian J Orthop 2018;52:140-6. [Google Scholar]
- 10.Yan Z, Xu J, Wu G, Zhen Y, Liao X, Zou F. Identification of key genes and pathways associated with gender difference in osteonecrosis of the femoral head based on bioinformatics analysis. J Musculoskelet Neuronal Interact 2023;23:122-30. [Google Scholar]
- 11.Zhao D, Cui D, Wang B, Tian F, Guo L, Yang L, et al. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone 2012;50:325-30. [Google Scholar]
- 12.Jindal K, Aggarwal S, Kumar P, Rathod P. Core decompression with bone marrow aspirate concentrate in post collapse avascular necrosis of hip: A systematic review and meta-analysis. J Clin Orthop Trauma 2021;17:78-87. [Google Scholar]
- 13.Chahla J, Mannava S, Cinque ME, Geeslin AG, Codina D, LaPrade RF. Bone marrow aspirate concentrate harvesting and processing technique. Arthrosc Tech 2017;6:e441-5. [Google Scholar]
- 14.Jeyaraman M, Bingi SK, Muthu S, Jeyaraman N, Packkyarathinam RP, Ranjan R, et al. Impact of the process variables on the yield of mesenchymal stromal cells from bone marrow aspirate concentrate. Bioengineering (Basel) 2022;9:57. [Google Scholar]
- 15.Cavallo C, Boffa A, de Girolamo L, Merli G, Kon E, Cattini L, et al. Bone marrow aspirate concentrate quality is affected by age and harvest site. Knee Surg Sports Traumatol Arthrosc 2023;31:2140-51. [Google Scholar]
- 16.Indrawattana N, Chen G, Tadokoro M, Shann LH, Ohgushi H, Tateishi T, et al. Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochem Biophys Res Commun 2004;320:914-9. [Google Scholar]
- 17.McCarrel T, Fortier L. Temporal growth factor release from platelet-rich plasma, trehalose lyophilized platelets, and bone marrow aspirate and their effect on tendon and ligament gene expression. J Orthop Res 2009;27:1033-42. [Google Scholar]
- 18.Tomaru Y, Yoshioka T, Sugaya H, Kumagai H, Aoto K, Wada H, et al. Comparison between concentrated autologous bone marrow aspirate transplantation as a hip preserving surgery and natural course in idiopathic osteonecrosis of the femoral head. Cureus 2022;14:e24658. [Google Scholar]
- 19.Shankar AN, Jayakumar T, Pranav NG, Jeyaraman M. Biological therapy for avascular necrosis of femoral head - A case report. J Orthop Case Rep 2023;13:27-31. [Google Scholar]
- 20.Baghdadi S, Chern I, Hanstein R, Mehraban Alvandi L, Fornari E. Femoral head core decompression and bone marrow concentrate injection in pediatric sickle-cell related avascular necrosis. J Pediatr Orthop 2023;43:e433-9. [Google Scholar]
- 21.Jeyaraman M, Muthu S, Jain R, Khanna M. Autologous bone marrow derived mesenchymal stem cell therapy for osteonecrosis of femoral head: A systematic overview of overlapping meta-analyses. J Clin Orthop Trauma 2021;13:134-42. [Google Scholar]
- 22.Jawad MU, Haleem AA, Scully SP. In brief: Ficat classification: Avascular necrosis of the femoral head. Clin Orthop Relat Res 2012;470:2636-9. [Google Scholar]
- 23.Kumar P, Sen R, Aggarwal S, Agarwal S, Rajnish RK. Reliability of modified Harris hip score as a tool for outcome evaluation of total hip replacements in Indian population. J Clin Orthop Trauma 2019;10:128-30. [Google Scholar]
- 24.Tomaru Y, Yoshioka T, Sugaya H, Kumagai H, Hyodo K, Aoto K, et al. Ten-year results of concentrated autologous bone marrow aspirate transplantation for osteonecrosis of the femoral head: A retrospective study. BMC Musculoskelet Disord 2019;20:410. [Google Scholar]
- 25.Hoogervorst P, Campbell JC, Scholz N, Cheng EY. Core decompression and bone marrow aspiration concentrate grafting for osteonecrosis of the femoral head. J Bone Joint Surg Am 2022;104:54-60. [Google Scholar]
- 26.Pawar N, Vaish A, Vaishya R. Core decompression and bone marrow aspirate concentrate injection for Avascular Necrosis (AVN) of the femoral head: A scoping review. J Clin Orthop Trauma 2022;24:101691. [Google Scholar]
- 27.Sugaya H, Yoshioka T, Tomaru Y, Kumagai H, Yamazaki M, Mishima H. An exploratory clinical trial for concentrated autologous bone marrow aspirate transplantation in the treatment of osteonecrosis of the femoral head. Eur J Orthop Surg Traumatol 2023;33:441-7. [Google Scholar]
- 28.Hauzeur JP, De Maertelaer V, Baudoux E, Malaise M, Beguin Y, Gangji V. Inefficacy of autologous bone marrow concentrate in stage three osteonecrosis: A randomized controlled double-blind trial. Int Orthop 2018;42:1429-35. [Google Scholar]
- 29.Cruz-Pardos A, Garcia-Rey E, Ortega-Chamarro JA, Duran-Manrique D, Gomez-Barrena E. Mid-term comparative outcomes of autologous bone-marrow concentration to treat osteonecrosis of the femoral head in standard practice. Hip Int 2016;26:432-7. [Google Scholar]