Blue Rubber Bleb Nevus Syndrome (BRBNS) is a rare syndrome with significant potential damage to articular cartilage, which can lead to hip dysplasia, coxarthrosis and, consequently, the need for hip arthroplasty at a young age.
Dr. David Popescu, Department of Orthopaedic and Trauma Surgery, Cliniques Universitaires Saint-Luc, 10, Avenue Hippocrate 1200 Bruxelles, 1200 Brussels, Belgium. E-mail: david.popescu@student.uclouvain.be
Introduction: Blue rubber bleb nevus syndrome (BRBNS) is a rare congenital vascular disorder that affects the venous system. Lesions are multiple and involved not only the skin and subcutaneous tissue but also muscles, joints and organs such as the gastrointestinal tract. At present, little is known regarding its potential orthopedic complications.
Case Report: We present a unique case of a patient with BRBNS displaying both intra-articular and extra-articular severe venous malformation (VM) of the hip. This extensive VM caused severe deformities in bone growth, mainly affecting the proximal femur, and impacted the muscular development of the gluteus medius and gluteus maximus. Its intra-articular extension, along with repeated secondary hemarthrosis, led to cartilaginous destruction. Consequently, the patient presented with significant coxa valga and developed acetabular dysplasia and subluxation of femoral head, during growth. In order to restore hip function and alleviate pain, the patient underwent a total hip arthroplasty (THA) at the age of 18. Discussion: The dysplastic changes in the hip joint observed in this case are attributed to the deleterious effects of VMs and coxa valga on joint anatomy and biomechanics. VMs induce recurrent hemarthrosis, leading to cartilage destruction and hip instability. Additionally, coxa valga alters hip biomechanics, exacerbating joint instability and accelerating wear. Surgical intervention with THA aimed to restore joint stability and function, although challenges arose due to anatomical complexities and limited prosthetic options.
Conclusion: This is the first reported case of hip dysplasia associated with BRBNS. This case shows the involvement of vascular malformation in the development of hip dysplasia leading to total hip arthroplasty. The surgical planning and technique must take the specificity of this pathology into account to get the best result possible for the patient. This case illustrates the importance of a multidisciplinary approach to treat patients with this specific syndrome and adds valuable information to the limited literature on orthopedic complications in BRBNS.
Keywords: Blue rubber bleb nevus syndrome, hip dysplasia, venous malformation, total hip arthroplasty, trendelenburg gait.
Blue rubber bleb nevus syndrome (BRBNS) is a rare congenital disorder (1/14,000 births) characterized by multiple ectatic-venous-like channels, known as venous malformations (VMs), mainly located in the skin and the gastro-intestinal (GI) tract [1]. Typically, patients are born with a large lesion and subsequently, developed small but multiple VMs located anywhere on the body. More lesions appear later in life. The underlyinh cause of BRBN is attributed to a double somatic mutation in the TIE2 gene [2]. The hallmark features of BRBNs includes [3-5]:
- Skin lesions: Characteristic skin lesions presents as bluish or purplish in color, with a rubbery texture, and varying in size. Lesions located on the palms and soles are pathognomonic of this syndrome.
- GI lesions: they can lead to severe GI bleeding, resulting in severe anemia and necessitating chronic blood transfusions.
- Besides the GI tract, BRBNS can affect other organs, such as the liver, spleen, lungs and brain. Involvement of these organs can lead to various symptoms depending on the extent and location of the lesions [6].
- Coagulation anomalies are commonly associated in patients with BRBNS: this coagulopathy is characterized by markedly elevated d-dimers and reduced fibrinogen levels, known as localized intravascular coagulopathy (LIC). Left untreated, LIC can progress to disseminated intravascular coagulopathy (DIC), that can cause severe chronic bleeding.
There is no consensus regarding the treatment of BRBNS. Clinical follow up with iron supplementation and blood transfusions if the patient becomes symptomatic has shown efficacy [4]. For symptomatic patients that have extended VM, surgical or endoscopic approach is considered with wedge resection of the affected part of the GI tract [4]. New mTOR inhibitors are reported to improve the symptoms of 95–99% of the patients [7]. This new oral drug has shown efficacy in reducing bleeding and pain. It is often used as a second line therapy if surgery is not sufficient. Hip dysplasia is characterized by abnormal development of the hip joint, resulting in instability and a heightened risk of dislocation. Notably, there is no direct correlation reported between developmental of dysplasia of the hip and BRBNS. However, VM is known to involve any type of tissues, such as nerve, muscle and joint and be responsible for cartilage destruction like seen in hemophilia patients. This is common in patients with knee VM unless they have been operated before destruction of the cartilage [8]. Hip involvement is not commonly seen. Muscle forces and joint reaction forces (JRFs) serve crucial roles in the dynamic stabilization of the hip joint, influencing femoral and acetabular development. An imbalance in these forces may contribute to pathological alterations. Specifically, the cranially displaced femoral head, indicative of dislocation, has been associated with abductor weakness. This weakness is significant as abductors are implicated in generating a medial force during hip development. The intricate interplay of muscle forces and JRFs in hip dynamics underscores their pivotal role in maintaining joint stability and preventing adverse morphological changes [9]. In rare situations, BRBNS can impact the muscles, making us think it might cause orthopedic problems. However, up to now, there have no been any reports of these specific types of complications linked to BRBNS. Treatment approaches for muscle weakness and hip dysplasia should be tailored to each individual’s specific condition. This may involve a combination of physical therapy, muscle-strengthening exercises and, in severe cases, surgical procedures [10].
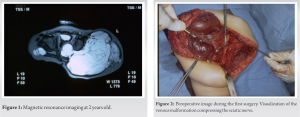
We report a 18-year-old patient affected by BRBNS who underwent total hip arthroplasty (THA). At the age of 2 years, the patient underwent surgical resection of an extensive venous vascular malformation involving the left gluteus maximus and the gluteus medius with extension into the hip (Fig. 1 and 2). This surgery was performed under prophylactic dosage of low-molecualr weight heparin to avoid peroperative decompensation of his consumptive coagulopathy and severe peroperative bleeding [11]. Postoperatively, rehabilitation facilitated partial motor recovery and the patient was able to sit and walk despite motor delay.
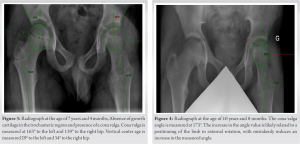
At the age of 4 years, a second surgery addressed another sizable angioma in the root of the left lower limb. At the age of 5 years, he was operated by varus proximal femoral osteotomy for coxa valga. However, the coxa valga progressively recurred. At the age of 7, the patient underwent additional surgery for the resection of a large-volume vascular malformation located in the gluteal muscles and left hallux. Postoperatively, intensive rehabilitation aimed at preserving musculature and restoring joint range of motion (ROM).
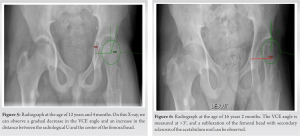
Vascular malformations probably induced a modification of the growth plates on the proximal femur. The deformity in coxa valga is likely related to a premature closure of the growth plate of the greater trochanter and overstimulation at the level of the femoral head despite the varus osteotomy done at the age of 4 years (Fig. 3). At the age of 8 years, the patient exhibited diminished hip rotation, with only 10° ROM achievable for both internal and external rotations. Consequently, intensive physiotherapy sessions were continued.
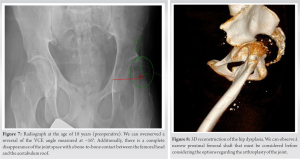
At the age of 10 years, there was no further orthopedic deterioration. While there was an improvement in pain levels during ambulation, a persistent gait abnormality remained. The decision at this stage was conservative, with no further orthopedic interventions (Fig. 4). During the following years, we noticed a progressive increase in the coxa valga and the presence of a more severe Trendelenburg gait forcing the patient to use crutches in order to walk. The patient was encouraged to continue physiotherapy and underwent regular examinations of the hip (Fig. 5, 6, 7, 8).
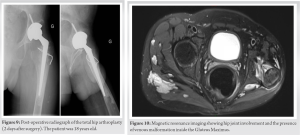
He was treated with mTor inhibitor (rapamycin) to reduce pain and bleeding. Under this medication, chronic bleeding stopped as well as abdominal pain. However, walking was still complicated due to the important subluxation of the femoral hip. The patient underwent a THA at the age of 18 years. Given the deformity of the proximal femur, it was not possible to consider the replacement of the joint through a minimal invasive approach (Fig. 8). We performed a THA using a posterior approach (Moore). This surgical approach allowed a wider opening and better exposition of the proximal femur in the event that a proximal femoral osteotomy should be considered. Considering the anatomy of the patient, we implanted a Continuum multihole screwed acetabular cup size 44 (Zimmer Biomet); cemented Versys CDH stem with a diameter of 9 mm (Zimmer Biomet), Highly Cross-linked polyethylene (HXLPE) liner, Biolox Delta head 28/0. In the postoperative period, the patient was instructed to mobilize with full weight-bearing using two crutches and to wear an anti-dislocation brace for 6 weeks due to the muscle weakness and the important risk of dislocation (Fig. 9).
Five-year postoperatively, the patient has not experienced any episodes of dislocation except once in the immediate postoperative period, the patient experienced a dislocation while being transferred from the operating table to their bed, necessitating an open reduction procedure. He walks with the assistance of one crutch, primarily due to knee pain. There is no compilation of the THA to this day.
Hip dysplasia can be categorized utilizing the Crowe [12] classifications, this dysplasia corresponds to a Type 2 at the timing of the surgery. This dysplasia evolved from a Type 1 during childhood caused by repeated damage to the hip joint that could be attributed to two mechanisms. First the presence of intra-articular VM with possibly repeated hemarthrosis [13]. Recurrent hemarthosis has a toxic effect on the proteoglycans growth. Studies shows that when cartilage is exposed to blood, it undergoes harmful changes very quickly, suggesting that even just one instance of bleeding within a joint can be harmful. The damage caused by this blood exposure seems to be long-lasting, as it significantly reduces the synthesis of proteoglycans—the building blocks of cartilage. This suppression of proteoglycan synthesis persists for as long as 20 days after only a brief, 4-day exposure to blood. The physiopathology of the destruction of proteoglycans is a combination of multiple factors [13, 14]:
- Oxygen Metabolites and Proteoglycan Destruction: Oxygen metabolites, such as superoxide (O2-) and hydrogen peroxide (H2O2), are produced in significant amounts by activated immune cells, particularly monocytes and macrophages. Chondrocytes, the cells found in cartilage, also produce these oxygen metabolites. The studies suggest that mononuclear cells and red blood cells play a role in inducing damage to cartilage [14, 15].
- Irreversible Effects on Proteoglycan Synthesis: Exposure to blood, particularly with the combination of MNCs and RBCs, results in a sustained suppression of proteoglycan synthesis in cartilage. This suppression is practically irreversible, leading to a significant and long-lasting impact on the structural components of cartilage [14].
- Involvement of Iron: Red blood cells may provide catalytically active iron, leading to the production of short-lived hydroxyl radicals. Free iron molecules, when present in trace amounts, can catalyze the reaction between superoxide and hydrogen peroxide, forming highly toxic hydroxyl radicals [16].
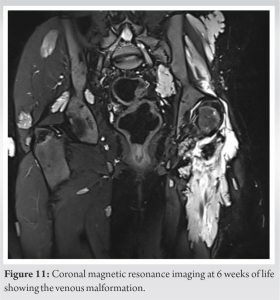
In our case, VMs were visible on the Magnetic resonance imaging (Figs. 10 and 11). Fig. 8 mainly shows the extent of vascular malformations affecting the muscle compartments around the hip joint. While Fig. 9, it illustrates the significance of intra-articular vascular malformations. We can assume that the joint has experienced recurrent episodes of hemarthrosis leading to cartilage alteration. The development of these intra-articular malformations, acting as a foreign body similar to a slow-evolving tumoral process, will exert a lateralizing pressure on the femoral head, leading to hip excentration. Other hip VM have been identified through more in-depth research. We observed that coxa valga is associated with other venous malformative diseases such as Klippel–Trénaunay syndrome (KTS) [17]. This suggests a link between hip development and vascularization. However, to date, we lack scientific evidence on this matter. The second is the coxa valga with femoral head excentration. In case of coxa valga, biomechanics of the hip are changed leading to changes in the mechanical stress of the joint. Moreover, the instability of the joint associated with coxa valga also increases the risk of hip joint instability [18]. There was a differential growth between greater trochanter and femoral head leading to coxa valga. This could be attributed to two mechanisms, epiphysiodesis of the greater trochanter growth plate by the vascular malformation and weakness of the altered Gluteus maximus during growth. Vascular malformations have caused significant destruction of the gluteus medius and gluteus maximus muscles in this patient, resulting in a lack of leverage and abduction movement. The thigh adductor muscles became predominant, leading to deformation of the limb in adduction and external rotation despite intensive physiotherapy. The adductor muscle movement and abductor insufficiency caused a progressive worsening of femoral head excentration, leading to its subluxation. The THA aimed to restore the center of rotation and improve the femoral offset to recover proper function of the gluteus medius and maximus muscles, which stabilize the pelvis during single-leg support. In this case, the most crucial aspect during the arthroplasty procedure, in addition to the femoral anatomical difficulty, was to minimize the medialization of the acetabulum and plan for the maximum possible offset to mitigate the risk of dislocation. In this patient, unlike a healthy hip, there is noticeable atrophy of the hip abductors, which increase instability and may predispose the patient to dislocations. With the femoral template being very narrow, we did not have the option to use a standard femoral stem, but we had to choose a stem specifically tailored to dysplasic femoral shaft. Unfortunately, this type of stem can only provide limited offset due to his narrow diameter; excessive offset induces a high risk of prosthetic neck fracture. Following the surgery and rehabilitation, the patient no longer exhibited visible signs of Trendelenburg gait [19]. However, he continued to use a cane on the side of the operated limb due to a vascular malformation at the knee, causing pain under load.
This is the first reported case of hip dysplasia associated with BRBNS. The joint and muscle involvement by the vascular malformation led to progressive subluxation of the femoral head with finally arthritis needing THA. The successful outcome post-THA underscores the effectiveness of tailored surgical interventions in managing advanced hip dysplasia in BRBNS. This is a typically example where we must consider not only the bone anatomy in the choice of the prosthesis and the surgical approach but also the muscular structures and vascular malformations, the patient morphotype, and their future evolution. This case adds valuable information to the limited literature on orthopedic complications in BRBNS, emphasizing the importance of considering musculoskeletal aspects in the comprehensive management of patients with this syndrome.
This case highlights the significant impact of vascular malformations on hip dysplasia and subsequent THA. It underscores the association between recurrent hemarthrosis due to intra-articular VMs and cartilage alteration, leading to hip joint instability and femoral head subluxation. Collaboration between orthopedic and vascular surgeons is crucial for comprehensive management, ensuring that orthopedic interventions are carefully planned to address musculoskeletal issues while considering the vascular aspects of the condition.
References
- 1.Martinez CA, Rodrigues MR, Sato DT, Silveira Júnior PP, Gama RF, Mattavelli CB, et al. Blue rubber bleb nevus syndrome as a cause of lower digestive bleeding. Case Rep Surg 2014;2014:683684. [Google Scholar]
- 2.Soblet J, Kangas J, Nätynki M, Mendola A, Helaers R, Uebelhoer M, et al. Blue rubber bleb nevus (BRBN) syndrome is caused by somatic TEK (TIE2) mutations. J Invest Dermatol 2017;137:207-16. [Google Scholar]
- 3.Jin XL, Wang ZH, Xiao XB, Huang LS, Zhao XY. Blue rubber bleb nevus syndrome: A case report and literature review. World J Gastroenterol 2014;20:17254-9. [Google Scholar]
- 4.Fishman SJ, Smithers CJ, Folkman J, Lund DP, Burrows PE, Mulliken JB, et al. Blue rubber bleb nevus syndrome: Surgical eradication of gastrointestinal bleeding. Ann Surg 2005;241:523-8. [Google Scholar]
- 5.Wassef M, Vanwijck R, Clapuyt P, Boon L, Magalon G. Vascular tumours and malformations, classification, pathology and imaging. Ann Chir Plast Esthet 2006;51:263-81. [Google Scholar]
- 6.Ballieux F, Boon LM, Vikkula M. Blue bleb rubber nevus syndrome. Handb Clin Neurol 2015;132:223-30. [Google Scholar]
- 7.Seront E, Van Damme A, Legrand C, Bisdorff-Bresson A, Orcel P, Funck-Brentano T, et al. Preliminary results of the European multicentric phase III trial regarding sirolimus in slow-flow vascular malformations. JCI Insight 2023;8:e173095. [Google Scholar]
- 8.Pireau N, Boon LM, Poilvache P, Docquier PL. Surgical treatment of intra-articular knee venous malformations: When and how? J Pediatr Orthop 2016;36:316-22. [Google Scholar]
- 9.Song K, Gaffney BM, Shelburne KB, Pascual-Garrido C, Clohisy JC, Harris MD. Dysplastic hip anatomy alters muscle moment arm lengths, lines of action, and contributions to joint reaction forces during gait. J Biomech 2020;110:109968. [Google Scholar]
- 10.Benedetti MG, Cavazzuti L, Amabile M, Tassinari E, Valente G, Zanotti G, et al. Abductor muscle strengthening in THA patients operated with minimally-invasive anterolateral approach for developmental hip dysplasia. Hip Int 2021;31:66-74. [Google Scholar]
- 11.Dompmartin A, Acher A, Thibon P, Tourbach S, Hermans C, Deneys V, et al. Association of localized intravascular coagulopathy with venous malformations. Arch Dermatol 2008;144:873-7. [Google Scholar]
- 12.Jawad MU, Scully SP. In brief: Crowe’s classification: Arthroplasty in developmental dysplasia of the hip. Clin Orthop Relat Res 2011;469:306-8. [Google Scholar]
- 13.Roosendaal G, Vianen ME, Marx JJ, Van Den Berg HM, Lafeber FP, Bijlsma JW. Blood-induced joint damage: A human in vitro study. Arthritis Rheum 1999;42:1025-2. [Google Scholar]
- 14.Stein H, Duthie RB. The pathogenesis of chronic haemophilic arthropathy. J Bone Joint Surg Br 1981;63B:601-9. [Google Scholar]
- 15.Bates EJ, Lowther DA, Johnson CC. Hyaluronic acid synthesis in articular cartilage: An inhibition by hydrogen peroxide. Biochem Biophys Res Commun 1985;132:714-20. [Google Scholar]
- 16.Roosendaal G, Vianen ME, Wenting MJ, van Rinsum AC, van den Berg HM, Lafeber FP, et al. Iron deposits and catabolic properties of synovial tissue from patients with haemophilia. J Bone Joint Surg Br 1998;80:540-5. [Google Scholar]
- 17.Zacharia B, Alex J, Rajmohan A. Klippel-Trénaunay syndrome and developmental coxa vara in the same limb: A case report with a review of the literature. Int J Angiol 2021;32:292-5. [Google Scholar]
- 18.Maquet P. Biomechanics of hip dysplasia. Acta Orthop Belg 1999;65:302-14. [Google Scholar]
- 19.Mitchell GP. Late congenital dislocation of the hip. Rev Chir Orthop Reparatrice Appar Mot 1981;67:241-7. [Google Scholar]