Late onset of hip pain after treatment of immune-related adverse events should be examined under suspicion of both remission of inflammatory arthritis and development of osteonecrosis of the femoral head.
Dr. Masaki Takao, Department of Orthopaedic Surgery, Ehime University Graduate School of Medicine, Japan. E-mail: takao.masaki.ti@ehime-u.ac.jp
Introduction: Immune checkpoint inhibitors (ICIs) are increasingly being used in the treatment of advanced metastatic and immunogenic cancers. However, these therapies could cause immune-related adverse events (irAEs), which require high-dose glucocorticoid administration.
Case Report: A 52-year-old man with metastatic renal cell carcinoma received ICI therapy. Two weeks later, he suffered from severe irAEs and received glucocorticoid therapy for 13 months. Twenty-one months after the initiation of glucocorticoid administration, he presented to us with bilateral hip pain and was diagnosed with bilateral osteonecrosis of the femoral head (ONFH).
Conclusion: IrAEs associated with ICI therapy might be an emerging underlying disease of ONFH.
Keywords: Immune checkpoint inhibitors, immune-related adverse events, glucocorticoid, osteonecrosis.
Immune checkpoint inhibitors (ICIs) are increasingly being used in the treatment of advanced metastatic and immunogenic cancers such as anti-cytotoxic T-lymphocyte antigen 4 antibody, anti-programmed death 1 (PD-1), and anti-programmed death ligand 1. In metastatic renal cell carcinoma (RCC), pembrolizumab (anti-PD-1 inhibitor) is recommended with axitinib which is an antivascular endothelial growth factor receptor-tyrosine kinase inhibitor (VEGFR-TKI) [1]. This combination therapy resulted in longer overall survival and progression-free survival [2]. ICIs showed uncontrolled collateral effects on the immune system that can lead to so-called immune-related adverse events (irAEs), which were observed in various normal organs. In common, irAEs appear in the liver, lung, endocrine gland, and skin [3, 4]. The musculoskeletal system is also a target of ICIs, and irAEs also appear as inflammatory arthritis in various forms which is similar to well-characterized inflammatory arthritis such as rheumatoid arthritis, psoriatic arthritis, or spondyloarthropathy [5]. Glucocorticoid is the mainstay of treatment for irAEs, which is known as a risk factor of osteonecrosis of the femoral head (ONFH), but there are few studies on the occurrence of ONFH during treatment of irAEs [6]. Here, we report a case of bilateral ONFH associated with glucocorticoid therapy for severe irAEs after ICIs treatment for metastatic RCC.
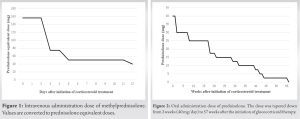
A 52-year-old man with no habits of alcohol consumption, smoking, glucocorticoid treatment, or immune diseases underwent a laparoscopic nephrectomy for a renal tumor. The diagnosis was “clear RCC expansive type pT3aN0M1,” stage Ⅳ. The combination therapy of pembrolizumab and axitinib was started. Two weeks later, irAEs appeared in the liver, kidney, thyroid (hypothyroidism), skin (rash), and eyes (uveitis). Laboratory tests were notable for aspartate aminotransferase of 392 U/L, alanine transaminase of 470 U/L, total-bilirubin of 2.2 mg/dL (direct-bilirubin of 1.1 mg/dL, indirect-bilirubin of 1.1 mg/dL), creatinine of 1.59 mg/dL, and C-reactive protein of 27.52 mg/dL. In thyroid function, thyroid-stimulating hormone of 10.56 μIU/mL was elevated, and free T3 of 1.62 pg/mL was slightly decreased. However, the count of white blood cell was normal and there were no findings of suspicion of infection throughout the body. In addition, the values of complements (CH50, C3, and C4) and antinuclear antibodies were slightly elevated, and there was no sign of diabetes and diabetes-related complications. It was grade 3 of the common terminology criteria for adverse events from the National Cancer Institute, which ranges from grade 1 to grade 5, which refers to mild, moderate, severe, life-threatening, or death in ascending order [7]. Promptly, this ICI therapy for metastatic RCC was stopped, and high-dose glucocorticoid therapy was started. Because of high body weight (125 kg, body mass index 38.3 kg/²), the patient received intravenous methyl-prednisolone administration of 125 mg/day for 3 days, 60 mg/day for 2 days, and 40 mg/day for 7 days (Fig. 1).
After that, oral administration of prednisolone (40 mg/day) was started and tapered down (Fig. 2).
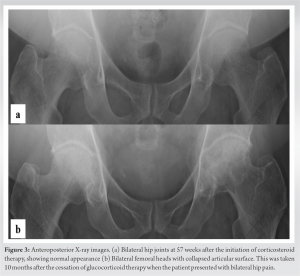
Overall, the maximum dose of prednisolone was 156.25 mg/day, the total dose was 6,582.25 mg and the duration of glucocorticoid treatment was 57 weeks and 5 days (Fig. 1 and 2). Axitinib treatment was restarted together last 5 months. Ten months after the cessation of glucocorticoid therapy, the patient presented to us with a complaint of bilateral hip pain. Radiography and magnetic resonance imaging (MRI) revealed bilateral ONFH (Fig. 3, 4, 5, 6).
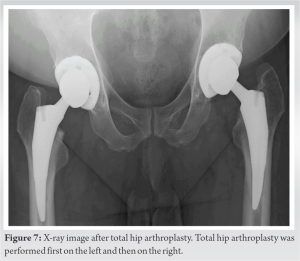
ONFH was classified as type C1 in the right hip and type C2 in the left hip according to the 2001 revised criteria for classification of ONFH by the Japanese Investigation Committee [8]. The stage of ONFH was stage 3A on the right and stage 3B on the left according to the 2019 revised version of the association research circulation osseous staging system of ONFH [9]. He underwent total hip arthroplasty (THA) for both sides with an interval of 10 months. (Fig. 7) 6 months after the last THA, 3½ years after laparoscopic nephrectomy, he lived a daily life with no disability.
The National Comprehensive Cancer Network categorized irAEs from 1 to 5 [7]. Severe irAEs (grade 3–4) reportedly appear in about 10% of anti-PD-1 inhibitors [10] and its incidence varies among the types of ICIs. systemic immune diseases such as systemic lupus erythematosus, anti-phospholipid syndrome, and anti-ANCA-related vasculitis are also forms of irAEs, which are well-known risk factors for the development of ONFH [5, 6]. The high-dose glucocorticoid therapy (intravenous methylprednisolone, 1–2 mg/kg/day) was mainly recommended for severe irAEs (grade 3–4). The relationship between ONFH and steroids has long been debated; however, high-dose glucocorticoid treatment for patients with systemic immune disorders is also recognized higher risk of development of ONFH [11]. With the increased use of ICIs for cancer treatment, the reported number of irAEs is increasing. irAEs can occur in any organ including the musculoskeletal system, but there are few reports on the development of ONFH [6]. As treatment with ICIs increases, we expect to see more cases of ONFH following treatment with irAEs. The mechanism of irAEs was not sufficiently understood; thus, direct effect of ICIs on blood flow in the femoral head is unclear. Anti-VEGFR-TKI inhibitor is recommended with ICIs in the treatment of metastatic RCC. Several studies have reported a relationship between VEGF polymorphisms and ONFH [12, 13], while the effect of anti-VEGFR-TKI inhibitors on blood flow in the femoral head is unclarified. The combination of ICIs and anti-VEGFR-TKI inhibitors for mRCC and high-dose glucocorticoid therapy for irAEs might influence the development of ONFH in the present case. IrAEs also appear as inflammatory arthritis such as rheumatoid arthritis, psoriatic arthritis, or spondyloarthropathy [6], which could present with hip pain. Reportedly, the median onset time of articular irAEs was 70 days after ICIs initiation. In a prospective MRI screening study of patients with SLE, the onset time of hip pain of ONFH ranged from 2 to 5 years after the initiation of glucocorticoid therapy [14]. Thus, late onset of hip pain after treatment of irAEs should be examined under suspicion of both remission of inflammatory arthritis and development of ONFH. One possible limitation is that image examination with X-ray or MRI was not performed before the onset of irAEs. Therefore, the timing of the ONFH development remains unknown. The onset of hip pain was 21 months after the initiation of glucocorticoid therapy, which suggested that the development of ONFH might develop after the onset of irAEs considering the time interval from the development of ONFH and the occurrence of collapse of the femoral head [15].
We experienced a case of bilateral ONFH that developed after glucocorticoid therapy due to irAEs associated with ICIs administration. Severe systemic irAEs require high-dose glucocorticoid therapy. IrAEs associated with ICI therapy might be an emerging underlying disease of ONFH.
Because high doses of glucocorticoids are used to treat adverse events associated with ICIs, irAEs associated with ICI therapy might be an emerging underlying disease of ONFH.
References
- 1.Ljungberg B, Albiges L, Abu-Ghanem Y, Bedke J, Capitanio U, Dabestani S, et al. European association of urology guidelines on renal cell carcinoma: The 2022 update. Euro Urol 2022;82:399-410. [Google Scholar]
- 2.Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019;380:1116-27. [Google Scholar]
- 3.Arnaud-Coffin P, Maillet D, Gan HK, Stelmes JJ, You B, Dalle S, et al. A systematic review of adverse events in randomized trials assessing immune checkpoint inhibitors. Int J Cancer 2019;145:639-48. [Google Scholar]
- 4.Gu L, Khadaroo PA, Su H, Kong L, Chen L, Wang X, et al. The safety and tolerability of combined immune checkpoint inhibitors (anti-PD-1/PD-L1 plus anti-CTLA-4): A systematic review and meta-analysis. BMC Cancer 2019;19:559. [Google Scholar]
- 5.Dang QM, Watanabe R, Shiomi M, Fukumoto K, Nobashi TW, Okano T, et al. Rheumatic immune-related adverse events due to immune checkpoint inhibitors-a 2023 update. Int J Mol Sci 2023;24:5643. [Google Scholar]
- 6.Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chávez A, Keegan N, Khamashta MA, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers 2020;6:38. [Google Scholar]
- 7.Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, et al. NCCN guidelines insights: Management of immunotherapy-related toxicities, version 1.2020. J Natl Compr Canc Netw 2020;18:230-41. [Google Scholar]
- 8.Sugano N, Atsumi T, Ohzono K, Kubo T, Hotokebuchi T, Takaoka K. The 2001 revised criteria for diagnosis, classification, and staging of idiopathic osteonecrosis of the femoral head. J Orthop Sci 2002;7:601-5. [Google Scholar]
- 9.Yoon BH, Mont MA, Koo KH, Chen CH, Cheng EY, Cui Q, et al. The 2019 revised version of association research circulation osseous staging system of osteonecrosis of the femoral head. J Arthroplasty 2020;35:933-40. [Google Scholar]
- 10.Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: A systematic review. Ann Oncol 2017;28:2377-85. [Google Scholar]
- 11.Powell C, Chang C, Naguwa SM, Cheema G, Gershwin ME. Steroid induced osteonecrosis: An analysis of steroid dosing risk. Autoimmun Rev 2010;9:721-43. [Google Scholar]
- 12.Liu Y, Zhang Z, Liu S, Su X, Zhou S. Association between VEGF-634G/C polymorphism and osteonecrosis of the femoral head susceptibility: A meta analysis. Int J Clin Exp Med 2015;8:10979-85. [Google Scholar]
- 13.Ma W, Xin K, Chen K, Tang H, Chen H, Zhi L, et al. Relationship of common variants in VEGFA gene with osteonecrosis of the femoral head: A Han Chinese population based association study. Sci Rep 2018;8:16221. [Google Scholar]
- 14.Sugano N, Ohzono K, Masuhara K, Takaoka K, Ono K. Prognostication of osteonecrosis of the femoral head in patients with systemic lupus erythematosus by magnetic resonance imaging. Clin Orthop Relat Res 1994;305:190-9. [Google Scholar]
- 15.Ito H, Matsuno T, Omizu N, Aoki Y, Minami A. Mid-term prognosis of non-traumatic osteonecrosis of the femoral head. J Bone Joint Surg Br 2003;85:796-801. [Google Scholar]