In a patient with true-positive loss of neuromonitoring, sequential imaging and pre-operative neurophysiology including MagStim should be used in deciding when to proceed with revision surgery and conservative surgical techniques should be used to minimize risk in an already compromised spinal cord.
Dr. Athanasios I. Tsirikos, Consultant Orthopaedic and Spine Surgeon, Honorary Clinical Senior Lecturer - University of Edinburgh, Scottish National Spine Deformity Centre, Royal Hospital for Children and Young People, 50 Little France Crescent, Edinburgh Bioquarter, EH16 4TJ, UK. E-mail: atsirikos@hotmail.com
Introduction: Multimodal intraoperative neuromonitoring (IOM) is essential in scoliosis surgery. This is affected by misplaced instrumentation, cord trauma, hemodynamic instability, and anesthesia. We present an irreversible loss of IOM without identifiable intra-operative cause to highlight its occurrence and discuss post-operative investigations and management.
Case Report: A 14-year-old girl with adolescent idiopathic scoliosis, no co-morbidities, and normal spinal magnetic resonance imaging (MRI) underwent posterior spinal fusion. During screw placement, bilateral motor evoked potentials (MEPs) and right somatosensory evoked potentials (SSEPs) were lost in the legs. All screws were removed with no evidence of cortical breach. Left leg responses gradually improved, but there was no recovery of right leg SSEPs or MEPs. Subsequently, the procedure was abandoned. The patient had reduced right leg strength (3/5) and sensation with the left leg was normal. Immediate post-operative spinal MRI identified no abnormality. Computed tomography (CT) showed no cortical breach with satisfactory pedicle screw tracts. Repeat MRI (day 7) showed high T2-signal within the cord at T11 indicating ischemia. Gradual neurological recovery occurred and on day 15, repeat neurophysiology found reproducible SSEPs and MagStim MEPs. The patient underwent revision posterior fusion with single rod correction without complication and IOM was maintained. By day 24, the patient had 5/5 power and normal sensation in both legs. Good scoliosis correction was achieved and maintained at 3-year follow-up.
Conclusion: This patient represents a vascular event affecting the lower spinal cord and highlights the role of sequential imaging and pre-operative neurophysiology including MagStim in deciding when to proceed with revision surgery while reducing risk using conservative techniques.
Keywords: Intraoperative neuromonitoring, Idiopathic scoliosis, Transcranial magnetic stimulation
Spinal cord injury is one of the most devastating complications associated with corrective scoliosis surgery with a reported incidence of 0.3–1.4% [1, 2]. With the introduction of intra-operative multimodal neuromonitoring, the frequency of such neurological complications has reduced significantly [3]. While somatosensory evoked potential (SSEPs) are useful in detecting dorsal column sensory function, MEPs play a major role in assessing the descending motor tracts of the spinal cord. This combined approach increases the sensitivity and specificity of identifying a neurological event during deformity correction [4]. The loss of multimodal intraoperative neuromonitoring (IOM) signals can be the result of several factors such as misplaced instrumentation, correction maneuvers, cord trauma, hemodynamic instability, and anesthetic agents. The operating team should be alerted and all such events should be addressed using an algorithm-based protocol [5]. Irreversible loss of signals may warrant performing a Stagnara wake-up test, removal of spinal instrumentation, or abandoning the procedure [6]. Although, pre-existing guidelines clearly outline the intra-operative management in events with loss of IOM signals, post-operative management and return to theater after such events is not reported in the literature. We present a case with irreversible loss of IOM signals without identifiable intra-operative cause to highlight its occurrence, as well as describe the need for post-operative investigations and further management with a special emphasis on the role of pre-operative SSEPs and transcranial magnetic stimulation (MagStim) MEPs.
A 14-year-old girl with adolescent idiopathic thoracic and lumbar scoliosis (Lenke 3C-) was planned to undergo a posterior instrumented fusion (PSF) from T4-L3. She had a normal pre-operative neurological examination and no medical co-morbidities. Pre-operative imaging included a spinal magnetic resonance imaging (MRI) scan that excluded abnormalities of the neural axis.
During anesthesia induction, propofol targeted controlled infusion and a bolus of atracurium (0.5 mg/kg) were used. Maintenance was achieved using infusion of propofol and remifentanil and ventilation with an air/oxygen mixture. Baseline cortical and cervical SSEPs along with MEP traces from tibialis anterior (TA), abductor halluces (AH), rectus femoris (RF), and abductor digiti minimi muscles were obtained after positioning the patient prone on the surgical table.
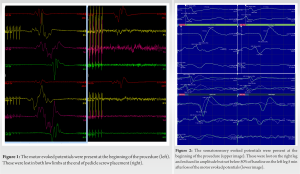
During instrumentation placement, the mean arterial pressure (MAP) was raised to a minimum of 65 mmHg. The pedicles targeted for pedicle screws were T10-L3 on the left and L2–L3 on the right side. The pedicle screws were placed from distal to proximal. At the end of pedicle screw placement (last screw positioned at left T10), the MEPs in both lower limbs were lost (Fig. 1). In accordance with the standard checklist [6], the immediate response included the reversal of the last surgical step that is the removal of left T10 and T11 pedicle screws followed by optimization of MAP, core body temperature and ruling out any anesthetic, positional or technical (electrodes and connections) causes of loss of MEP signals. Within 5 min, the SSEPs were also lost on the right leg and reduced in amplitude but not below 50% of baseline on the left leg (Fig. 2). All remaining pedicle screws were removed and the pedicle tracts had no cortical breach at any level. Elevation of MAP over 80 mmHg (hemodynamic optimization) resulted in gradual recovery of IOM traces (both MEPs and SSEPs) on the left side over a period of 30 min; however, the right side showed no improvement. As there was no improvement in the SSEP and MEP responses on the right side, the operation was abandoned.
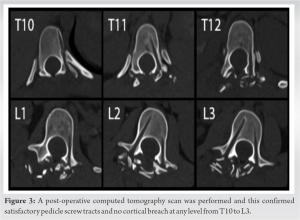
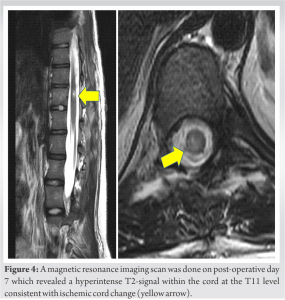
Upon immediate post-operative neurological assessment, the patient had reduced muscle strength and sensation in her right leg whereas the left leg showed intact neurology. Muscle power scores (using ASIA assessment for spinal cord injury) on the right low limb were 3/5 for hip flexion, knee extension and long toe extension, 2/5 for ankle dorsiflexion, and 4/5 for ankle plantar flexion. Light touch sensation L2-S2 was reduced and pinprick sensation was normal across all dermatomes on the right leg. An immediate post-operative CT scan was performed which confirmed no cortical breach at any level from T10-L3 (Fig. 3) and a spinal MRI scan revealed no abnormality. A repeat MRI scan was done on day 7 which revealed a hyperintense T2-signal within the cord at the T11 level consistent with ischemic cord change (Fig. 4). Treatment following the initial procedure focused on airway clearance along with intense physiotherapy including muscle strength and ROM exercises in bed. The patient was fitted with a spinal brace and mobilized out of bed using a Sara Stedy transfer aid.
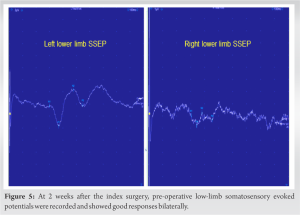
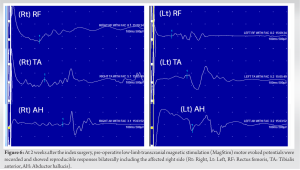
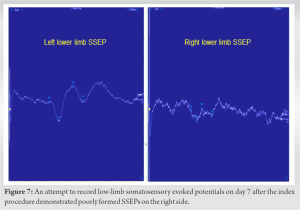
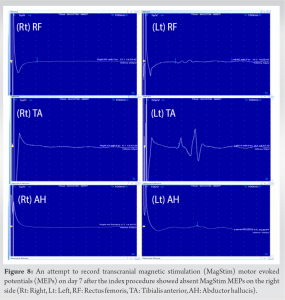
Gradual neurological improvement was observed over the next 2 weeks. Neurological examination on post-operative day 15 showed muscle strength 4/5 for hip flexion, knee extension, and long toe extension with normal power in all other muscle groups and normal sensation in the right leg. To determine the feasibility of intra-operative neuromonitoring, pre-operative low-limb SSEPs and MagStim MEPs were recorded and showed consistent responses bilaterally including the affected right side (Fig. 5 and 6). It should be noted that a similar attempt to record low-limb SSEPs and MagStim MEPs on day 7 after the index procedure did not yield reproducible traces on the right side, which resulted in a decision to delay the revision surgery for an additional week (Fig. 7 and 8). For MagStim MEPs, a 90 mm “Double Cone Coil” with a figure of eight configurations (Magstim Bistim 2002 unit, the Magstim Company UK, Ltd.,) was used to stimulate the motor strip of the cortex and the MEPs were recorded using the Medelec Synergy System (10 channel EMG/EP Plinth System; Viasys Healthcare GmbH), from stick-on electrodes on right and left TA, AH, and RF. For SSEPs, electrical stimulation of the posterior tibial nerve at the ankle took place through pre-gelled surface electrodes and responses were recorded from a single stick-on electrode at the C2 spinous process.
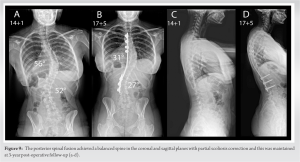
The patient underwent revision PSF with single rod instrumentation correction and autologous rib graft supplemented by allograft bone. No complication was encountered and the IOM remained stable throughout surgery. By day 24, the patient had 5/5 muscle power and normal sensation in both legs. A spinal brace was fitted to provide additional trunk support during bone healing. Elective re-grafting to reinforce the fusion mass was performed 12-month post-surgery using iliac crest bone and during this procedure, the instrumented levels were found to be fused. At this stage, the patient had returned to normal physical activities including sports with no limitations and no complaints of her back. A balanced spine was achieved with partial scoliosis correction and this was maintained at 3-year post-operative follow-up (Fig. 9).
Intra-operative loss of neuromonitoring signals often signifies cord at risk or spinal cord injury during deformity correction [3]. Management of a patient after permanent loss of intra-operative signals and subsequent neurological deficit can pose a difficult dilemma to the surgeon who has to make critical decisions that will decisively affect the outcome of treatment [4]. An immediate post-operative CT scan is a useful investigation to rule out pedicle screw malposition [6]. In addition, a post-operative MRI may be helpful to diagnose the level and cause of cord insults such as ischemia or mechanical compression. In the case described above, the MRI scan showed an area of hyperintensity in the T2-weighted images consistent with a vascular injury to the cord. It is of note that this signal abnormality was not visible in the immediate MRI performed in the evening after the initial procedure. Upon reviewing the literature, we found three case studies with delayed onset of neurological deficits in the post-operative period due to cervical vascular cord injury after thoracic deformity correction [7-9]. Dapunt et al. reported cervical cord injury due to venous congestive myelopathy caused by arteriovenous shunting from the hypervascular T4 body to the epidural venous plexus as seen in MR angiogram [9]. Quinonez et al. reported pressure on the cord from concave pedicles resulting in cord compression and tetraplegia with neurological recovery after removal of the pedicles [10]. Other authors have hypothesized tension on the spinal cord or vascular system and occult pre-operative injury or anatomic abnormalities, autonomic irregularities, and hypotension as possible causes for unexplained vascular injuries to the cord in patients undergoing deformity correction [8]. We believe that MAP fluctuations compounded by the complex vascular anatomy may have accounted for a vascular injury affecting the cord in our patient. Although several authors have reported delayed onset of neurological deficits after deformity correction [11,12], a vascular complication at the early instrumentation stage has not been reported to the best of our knowledge. The timing of return to theaters, as well as the ability to obtain consistent and reproducible neuromonitoring traces, is critical. In addition to the neurological recovery, we recommend the use of awake transcranial magnetic stimulation MEPs along with SSEPs as an indicator for planning the revision surgery for two reasons. First, it tests the pathways in advance which allow to determine the feasibility of monitoring which in turn guides patient consent. Second, it offers a baseline for later comparisons both in a clinical and medicolegal setting [13-15]. Several studies have shown the role of transcranial magnetic stimulation in the assessment of integrity and function of motor pathways in awake patients as it is painless and effective in generating MEPs which can be recorded from the specific muscle groups [13,14]. Furthermore, the latency and central motor conduction time of magnetic MEPs recorded in an awake patient have been shown to be congruent with electrical MEPs recorded in an anesthetized patient [15]. Therefore, awake MEPs can help predict the timing and the feasibility of IOM support during revision surgery. When planning the revision procedure, a more conservative approach should be adopted as a compromised spinal cord is more susceptible to injury even with minor maneuvers. In addition, if a specific area of cord insult is identified in the post-operative imaging, instrumentation at that level should be avoided when possible. While dual rod in situ fixation is the standard of care [16], we performed left-sided single-rod instrumentation in this case with partial scoliosis correction to avoid any instrumentation on the affected side. The single-rod instrumentation technique is an effective alternative to achieve satisfactory deformity correction with a low rate of complications in patients with specific indications and severe underlying medical conditions [16]. Further, an additional elective re-grafting procedure was done to ensure fusion and avoid any chances of pseudoarthrosis.
Avoiding neurological injury during deformity correction is imperative. Unexplained neurological damage can occur despite adequate clinical practice. In the case described above, abandoning the primary surgery and ensuring adequate recovery time to the cord have likely played a crucial role in preventing a catastrophic neurological complication. Return to theater can be guided clinically by the timing of neurologic recovery and through the use of awake pre-operative neuromonitoring. In the presence of a spinal cord at risk, the surgical priorities should be modified and a more conservative approach should be adopted to reduce the risk of repeat neural insults resulting in potentially permanent neurological deficits while achieving a satisfactory surgical result.
In a patient with true-positive loss of neuromonitoring, sequential imaging and pre-operative neurophysiology including MagStim should be used in deciding when to proceed with revision surgery and conservative surgical techniques should be used to minimize risk in an already compromised spinal cord.
References
- 1.Pahys JM, Guille JT, D’Andrea LP, Samdani AF, Beck J, Betz RR. Neurologic injury in the surgical treatment of idiopathic scoliosis: Guidelines for assessment and management. J Am Acad Orthop Surg 2009;17:426-34. [Google Scholar]
- 2.Chen J, Shao X, Sui W, Yang JF, Deng YL, Xu J, et al. Risk factors for neurological complications in severe and rigid spinal deformity correction of 177 cases. BMC Neurol 2020;20:433. [Google Scholar]
- 3.Kundnani VK, Zhu L, Tak HH, Wong HK. Multimodal intraoperative neuromonitoring in corrective surgery for adolescent idiopathic scoliosis: Evaluation of 354 consecutive cases. Indian J Orthop 2010;44:64-72. [Google Scholar]
- 4.Ifthekar S, Ahuja K, Mittal S, Yadav G, Goyal N, Rana AS, et al. Role of intraoperative neuromonitoring in spine surgery: A retrospective study. J Med Evid 2020;1:80-4. [Google Scholar]
- 5.Tsirikos AI, Duckworth AD, Henderson LE, Michaelson C. Multimodal intraoperative spinal cord monitoring during spinal deformity surgery: Efficacy, diagnostic characteristics, and algorithm development. Med Princ Pract 2020;29:6-17. [Google Scholar]
- 6.Vitale MG, Skaggs DL, Pace GI, Wright ML, Matsumoto H, Anderson RC, et al. Best practices in intraoperative neuromonitoring in spine deformity surgery: Development of an intraoperative checklist to optimize response. Spine Deform 2014;2:333-9. [Google Scholar]
- 7.Samtani RG, Bernatz JT, Halanski MA, Noonan KJ Cervical spine injury following thoracic spinal fusion for adolescent idiopathic scoliosis. Cureus 2019;11:e5840. [Google Scholar]
- 8.Youlo ST, Merrick MT, Cassidy JA, Halanski MA. Cervical spinal cord injury after thoracic spinal instrumentation: A case series. Spine (Phila Pa 1976) 2013;38:E1704-8. [Google Scholar]
- 9.Dapunt UA, Mok JM, Sharkey MS, Davis AA, Foster-Barber A, Diab M. Delayed presentation of tetraparesis following posterior thoracolumbar spinal fusion and instrumentation for adolescent idiopathic scoliosis. Spine 2009;34:E936-41. [Google Scholar]
- 10.Quinonez A, Pahys JM, Samdani AF, Hwang SW, Cahill PJ, Betz RR. Complete paraplegia 36 h after attempted posterior spinal fusion for severe adolescent idiopathic scoliosis: A case report. Spinal Cord Ser Cases 2021;7:33. [Google Scholar]
- 11.Auerbach JD, Kean K, Milby AH, Paonessa KJ, Dormans JP, Newton PO, et al. Delayed postoperative neurologic deficits in spinal deformity surgery. Spine (Phila Pa 1976) 2016;41:E131-8. [Google Scholar]
- 12.Qiao J, Xiao L, Zhu Z, Xu L, Qian B, Liu Z, et al. Delayed postoperative neurologic deficit after spine deformity surgery: Analysis of 5377 cases at 1 institution. World Neurosurg 2018;111:e160-4. [Google Scholar]
- 13.Galloway GM, Dias BR, Brown JL, Henry CM, Brooks DA 2nd, Buggie EW. Transcranial magnetic stimulation--may be useful as a preoperative screen of motor tract function. J Clin Neurophysiol 2013;30:386-9. [Google Scholar]
- 14.Kimiskidis VK, Potoupnis M, Papagiannopoulos SK, Dimopoulos G, Kazis DA, Markou K, et al. Idiopathic scoliosis: A transcranial magnetic stimulation study. J Musculoskelet Neuronal Interact 2007;7:155-60. [Google Scholar]
- 15.Glasby MA, Tsirikos AI, Henderson L, Horsburgh G, Jordan B, Michaelson C, et al. Transcranial magnetic stimulation in the semi-quantitative, pre-operative assessment of patients undergoing spinal deformity surgery. Eur Spine J 2017;26:2103-11. [Google Scholar]
- 16.Tsirikos AI, Loughenbury PR. Single rod instrumentation in patients with scoliosis and co-morbidities: Indications and outcomes. World J Orthop 2018;9:138-48. [Google Scholar]