Periprosthetic joint infection may be caused by swimming activities in the postoperative period.
Dr. Albagly Aviram, Department of Orthopedic Surgery, Bnai Zion Medical Center, Haifa, Israel. E-mail: aviram50@gmail.com
Introduction: Periprosthetic joint infection (PJI) is a severe complication of total joint arthroplasty (TJA), occurring in approximately 1–2% of primary procedures. These infections, characterized by an overall weighted mean incidence of 0.97% for total hip arthroplasty (THA) and 1.03% for total knee arthroplasty (TKA), pose significant challenges for patients and health-care providers.
Case Report: The authors present two intriguing case reports. The first involves a 64-year-old male who underwent TKA 17 months prior and presented to our outpatient clinic with clinical symptoms and laboratory findings consistent with PJI. Subsequent Streptococcus dysgalactiae culture results confirmed the diagnosis. The second case involves a 51-year-old male who presented to our outpatient clinic 2 weeks after undergoing THA, with clinical symptoms consistent with infected hematoma, which was later confirmed with positive Staphylococcus lugdunensis culture results and another infected process, occurring 3 months after the initial procedure, confirmed by positive Group B Streptococcus (Streptococcus agalactiae). Both patients reported swimming in a recreational swimming pool before and after the procedures and during the follow-up period, approximately 2–3 times a week. Those patients reported resuming their recreational swimming activities just a few days after the procedures. Although as part of post-operative wound care, we instruct all of our patients not to get the wound wet for the 1st days after surgery, to our knowledge swimming activities are not included in the various risk factors for developing a PJI, nor are the bacteria mentioned above associated with aquagenic infection.
Conclusion: Although swimming activity has not been identified as a known risk factor for PJI, it is important for physicians to maintain a high clinical suspicion for delayed or late infections in patients who engage in swimming activities. Further investigations are warranted to determine the potential role of recreational swimming activities as a risk factor for PJI after TJA.
Keywords: Periprosthetic joint infection, total knee arthroplasty, total hip arthroplasty, total joint arthroplasty, swimming activity, peri-prosthetic joint infection
Periprosthetic joint infection (PJI) is a serious complication of total joint arthroplasty (TJA), occurring in approximately 1–2% of primary procedures [1]. These infections, characterized by an overall weighted mean incidence of 0.97% for total hip arthroplasty (THA) and 1.03% for total knee arthroplasty (TKA), pose significant challenges for patients, and health-care providers [1]. Streptococcus dysgalactiae (SD) is a Gram-positive, beta-hemolytic coccal bacterium that can cause PJI following TJA. SD has two primary sub-species: SD subspecies equisimilis (SDSE), which is commonly associated with PJI, and SD subspecies dysgalactiae, which is rarely implicated in human infections. Remarkably, in a multicenter retrospective study, a substantial 17.1% of patients who underwent TKA and THA reported prosthetic joint infection with SD [2]. SDSE, classified as Group C and Group G streptococci, primarily affects humans and manifests in various clinical forms, ranging from superficial skin infections to severe necrotizing fasciitis and bacteremia [3]. Notably, there have been two case reports describing SDSE as the causative agent in PJIs. One report highlighted a patient who developed PJI with SDSE 3 years after TKA [4], while the other described a patient who experienced two episodes of PJI due to SDSE within a 9-month interval, following bilateral knee arthroplasties performed approximately 4 years apart [5]. Group B Streptococcus (GBS), also known as Streptococcus agalactiae, is a gram-positive, beta-hemolytic cocci bacterium primarily recognized as a significant pathogen in infants and pregnant women. However, GBS infections have become a growing concern among non-pregnant adults and the elderly [6]. In the context of PJI, Strep. species represent the second most common pathogens, accounting for 9–10% of all PJI cases [7]. In this group of PJI with Strep. Species, a multicenter retrospective study has shown that GBS was the most identified Strep. Species, with 38.6% of the Strep. PJI infections, followed by Strep. Dysgalactiae with 17.1% [2]. GBS infections are believed to predominantly spread hematogenously from a distant primary focus of infection [8]. While previous studies have indicated high remission rates for streptococcal PJI [9], GBS infections have been associated with a higher risk of relapse. Compared to the conservative approach of debridement and implant retention, treatment with debridement, antibiotics, and implant retention has demonstrated better outcomes for GBS-associated PJI [2]. Staphylococcus lugdunensis (SL) is a Gram-positive, catalase-positive, coagulase-negative cocci, and accounts for up to one-third of bone and joint infections, primarily related to hardware infections [10]. PJI caused by SL is particularly challenging to treat, with a multicentric cohort study reporting a 20% failure rate in the treatment of 111 patients with SL-associated PJI [11]. In this report, we present two intriguing case reports. The first involves a 64-year-old male who underwent TKA 17 months prior and presented to our outpatient clinic with clinical symptoms and laboratory findings consistent with PJI. Subsequent SD culture results confirmed the diagnosis. The second case involves a 51-year-old male who presented to our outpatient clinic 2 weeks after undergoing THA with clinical symptoms consistent with infected hematoma, which was later confirmed with positive SL culture results. On completing the appropriate treatment, which consisted of surgical debridement of the wound followed by a 6-week course of continuous antibiotic treatment through venous catheterization, he was discharged home, and 3 months after the initial procedure, the patient returned to our department with clinical symptoms and laboratory findings suggesting another infected process. A positive GBS (S. agalactiae) culture confirmed the suspicion. Both patients reported engaging in recreational swimming activity in a pool about a few days after the procedure and during the follow-up period. However, to the best of our knowledge, swimming activities are not included among the various risk factors for developing PJI [12, 13]. Our objective is to thoroughly examine the medical records of these patients, provide a comprehensive description of the post-procedure clinical scenario, and investigate a potential link between swimming pool activities and PJI following TJA. To accomplish this, we have carefully analyzed clinic summaries, discharge summaries, and operation reports, which serve as crucial evidence in describing these case reports.
First case
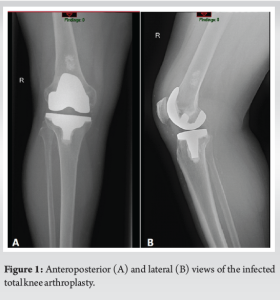
A 64-year-old male had a history of left TKA 2 years prior and sought medical attention at our outpatient clinic 17 months after undergoing right TKA. Past medical history includes benign essential hypertension, mixed hyperlipidemia, and chronic ischemic heart disease. He complained of pain, redness, and localized heat sensation around his right knee and calf which started 3 days before presentation, accompanied by fever with a recorded temperature of 39°C. Interestingly, the patient disclosed his regular visits to a swimming pool, which began a few days after the procedure, which became a notable point of investigation. The patient reveals that this recreational swimming activity was present also while he underwent the TKA on the left 2 years prior, with no complication presented at the time.
Clinical presentation and diagnosis
On physical examination, we observed pain, redness, swelling, and increased warmth in the right knee, along with limited range of motion (ROM) and weight-bearing capacity. The patient denied any insect bites or knee trauma, further emphasizing the potential influence of swimming activities. Admission to the orthopedic department was warranted, and a blood test revealed elevated levels of leukocytes (23.37 × 109 cells per liter on arrival and 27.56 × 109 cells per liter on the following day) and C-reactive protein (CRP) (170.2 mg/L on arrival and 235.4 mg/L on the following day), indicative of an inflammatory response. Notably, renal function, including glomerular filtration rate, and liver enzyme levels were within the normal range.
Treatment and progress
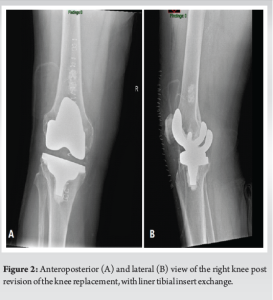
With the patient’s consent, following a plain radiograph of the infected knee (Fig. 1), an arthrocentesis of the right knee was performed on the following day. The aspiration yielded a purulent exudate, confirming the presence of an infection. Subsequently, the patient underwent surgical intervention, including incision and drainage (I+D) with polyethylene liner tibial insert exchange (Fig. 2). The aspirated fluid was sent for culture, and the results revealed a positive finding for SD. This discovery prompted consultation with the infectious diseases department, initiating an antibiotic therapy regimen consisting of one million units of penicillin G administered 6 times a day, with a recommended treatment duration of 4–6 weeks. During the patient’s 16-day admission to the department, a notable improvement in his condition was observed. Subsequent blood tests showed no leukocytosis, and CRP levels declined to 49.3 mg/L on the day of discharge. Consequently, the antibiotic treatment was transitioned to third-generation Cephalosporin (Ceftriaxone Sodium, 2 g/day) administered through venous catheterization, an approach that is commonly employed in our department.
Follow-up and long-term outcome
Two weeks after discharge, the patient returned for follow-up at our outpatient clinic. He reported slight residual pain, primarily experienced during the transition from sitting to standing. However, a physical examination revealed no signs of redness or localized heat in the right knee, and the patient exhibited a full ROM. Laboratory tests did not indicate an increase in inflammation markers, and X-ray imaging confirmed the satisfactory positioning of the knee implant. After completing the prescribed 6-week antibiotic treatment regimen, the patient’s condition continued to improve. At present, at the 18-month mark post-operation, the patient reports significant improvement in his clinical symptoms. Laboratory tests reveal no abnormal findings, with a leukocyte count of 7.0 × 109 cells per liter and CRP levels of 10.4 mg/L. Although the patient still experiences slight sensations of pain, primarily during stair climbing, his ROM has improved gradually, with full ROM achieved by 18-month post-operation.
Second case
A 51-year-old male patient with no medical history experienced complications following THA in his right knee. The patient presented to our department with post-operative swelling and pain, despite no abnormal findings on X-ray examination. Of particular interest, the patient revealed his involvement in recreational swimming activities, prompting us to investigate the potential association between swimming and the development of complications.
Clinical presentation and diagnosis
On physical examination, we noted pain, limited ROM, redness around the surgical scar without secretion, and swelling with induration around the right thigh. The patient denied any trauma to the area but admitted to regular swimming in a pool. Laboratory work revealed an elevated leukocyte count of 11.80 × 109 cells per liter and a CRP level of 26 mg/L, indicating an inflammatory response.
Treatment and progress
With the patient’s consent, a surgical incision and drainage (I+D) was performed the next day, during which an infected hematoma was revealed and drained. After thoroughly washing the infected area, aspiration of the joint revealed clear fluid with no puss. Due to that, a decision was made to not open the fascia, and liner exchange was not preformed. Two days later, the culture of the aspirate fluid was negative, however, the culture results of the hematoma yielded positive for SL and Enterobacter Cloacae. In consultation with the infectious diseases department, antibiotic treatment with 1st-generation Cephalosporin (Cefazolin IV, 2 g, 3 times/day) was initiated. Following surgery and treatment, leukocyte count and CRP levels gradually decreased to normal values. The patient was discharged with a 6-week course of continuous antibiotic treatment through venous catheterization.
Follow-up and long-term outcome
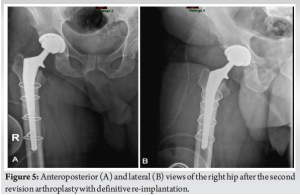
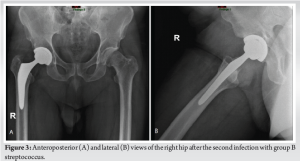
During the 6-week follow-up visit, the patient showed no signs of pain, redness, or limited ROM. Laboratory tests revealed a CRP level of 0.33 mg/L and an erythrocyte sedimentation rate of 2 mm/h, indicating a favorable response to treatment. However, 3 months after the initial procedure, the patient returned with sudden pain, redness, and swelling at the surgical site in the right thigh, accompanied by general weakness, chills, and fever. Further inquiry revealed that the patient had resumed swimming in the pool after completing the previous antibiotic treatment. Additional investigation showed no reliable connection between the current infection and the previous one. A plain radiograph of the infected right hip was performed (Fig. 3). Given the clinical suspicion of necrotizing fasciitis, a CT scan of the pelvic and thigh regions was performed, which did not show evidence of gas in the soft tissues or abscess. Due to the possibility of disseminated infection, the patient was transferred to the operating room for lateral fasciotomy, arthrotomy, joint aspiration, and the application of vacuum-assisted closure therapy. The aspirated fluid culture revealed a positive finding for S. agalactiae. Antibiotic treatment was adjusted to include Penicillin (12 million units, 2 times/day) and Clindamycin (Dalacin, 600 mg, 3 times/day). With the patient’s condition showing signs of improvement, including laboratory results with decreased leukocyte count and CRP (8 × 109 cells/L and 99 mg/L, respectively), a two-stage revision of THA was scheduled. The first stage involved the transplantation of a cement spacer, which occurred 10 days after hospitalization, on completing 6 weeks of antibiotic treatment (Fig. 4). Forty-two days later, when the patient’s condition was clinically stable, with no fever, hemodynamically stable vital signs, and normal laboratory values, a decision was made, and the patient underwent the second stage of the revision (Fig. 5). The patient’s post-operative status remained satisfactory, with no limitations in ROM and successful achievement of full weight-bearing capacity after physiotherapy sessions. Final laboratory tests showed a leukocyte count of 7.49 × 109 cells per liter and a CRP level of 12 mg/L. Two and a half months after the initial hospitalization with the second infection, when the two-stage revision was completed, followed by the mentioned above IV antibiotic treatment, the patient was discharged in satisfactory condition, with a further 12-day course of antibiotic treatment using Rifampicin (Rifampin, 300 mg twice per day, PO).
Follow-up and long-term outcome
Regular follow-up appointments were scheduled every 4–5 months at our outpatient clinic. During the patient’s past visit, 14 months after the second revision procedure, he reported feeling well, with no pain or limitations in ROM. The surgical scar appeared normal, and laboratory tests did not indicate an increase in leukocyte count or CRP levels, suggesting a favorable long-term outcome.
PJI is a significant complication following TJA, with the highest rate of infections occurring within the first 2 years after surgery [14]. An early or delayed PJI is mostly attributed to microorganisms introduced during the surgical procedure, while contiguous spread from adjacent sites is the second mechanism by which PJI can be imitated [15]. Various risk factors, such as high body mass index, diabetes mellitus, history of steroid use, and history of osteoarthritis or rheumatoid arthritis, have been associated with an increased probability of PJI [12, 13]. In these case reports, we present two instances of PJI occurring in males, up to a year and a half after TJA, who admitted to engaging in swimming activity before and after their surgeries. The identified bacteria in these cases, SDSE and GBS, are well-known pathogens associated with PJI. SDSE possesses virulence determinants like Streptococcus pyogenes and exhibits factors crucial for tissue dissemination [3, 16]. GBS, predominantly spread through hematogenous routes, harbors various virulence factors, including capsular polysaccharides and beta-hemolysin [17]. Although the pathogenicity of these bacteria is well established, no literature evidence has established a reliable connection between swimming activity and PJI-causing bacteria [18]. Infection after TJA is classified as “early” when symptoms occur within 3 months, “delayed” when symptoms occur between 3 and 24 months, and “late” when presenting symptoms occur beyond 2 years. Early and delayed PJIs typically result from microorganisms introduced during surgery, while late presentations suggest hematogenous seeding from another body site [19]. However, hematogenous infections can occur even shortly after prosthesis insertion [20]. Superficial site infections (SSI) may progress to involve the prosthesis, offering a potential explanation for such infections [15]. Risk factors associated with SSI include patient-related and surgery-related factors, but swimming activity has not been identified as a risk factor [21]. Interestingly, a retrospective review noted higher infection rates after TJA during the summer months, particularly July, August, and September [22]. Although in those months, people are more active and outside, one must consider that swimming activities also tend to occur more frequently in those months. Our cases, for example, occurred in July (the first case) and May (the second case). Although the literature lacks sufficient data on the association between recreational swimming activity and PJI, based on these case reports, it is crucial for physicians to maintain a high clinical suspicion for delayed or late infections in patients who engage in swimming activities. Further investigations are warranted to determine whether swimming activity should be considered a potential risk factor for PJI after TJA
We presented two intriguing case reports of PJI following TJA in patients who engaged in swimming activity before and after their surgeries. These cases involved the presence of SDSE and GBS, known pathogens associated with PJI. Still, no literature evidence currently establishes a reliable connection between swimming activity and those microorganisms or PJI. The timing of infection and the absence of other known risk factors warrant further investigation. The identified bacteria, SDSE and GBS, possess virulence factors contributing to tissue dissemination and hematogenous spread, respectively. While PJI is typically categorized as early, delayed, or late based on the timing of symptoms, hematogenous infections can occur shortly after prosthesis insertion. SSI may progress to involve the prosthesis, potentially explaining these cases. Although swimming activity has not been identified as a known risk factor for PJI, it is important for physicians to maintain a high clinical suspicion for delayed or late infections in patients who engage in swimming activities. Interestingly, a retrospective review noted higher infection rates during the summer months when swimming activities tend to be more frequent. This observation raises the possibility of a seasonal association between swimming and PJI. The authors believe that further investigations are warranted to determine the potential role of recreational swimming activities as a risk factor for PJI after TJA.
It is crucial to expand our understanding of the relationship between swimming and PJI to guide patient education, preventive measures, and post-operative care. By elucidating this potential association, we can improve patient outcomes and develop targeted strategies to mitigate the risk of PJI in this specific patient population.
References
- 1.Springer BD, Cahue S, Etkin CD, Lewallen DG, McGrory BJ. Infection burden in total hip and knee arthroplasties: An international registry-based perspective. Arthroplast Today 2017;3:137-40. [Google Scholar]
- 2.Mahieu R, Dubée V, Seegers V, Lemarié C, Ansart S, Bernard L, et al. The prognosis of streptococcal prosthetic bone and joint infections depends on surgical management-A multicenter retrospective study. Int J Infect Dis 2019;85:175-81. [Google Scholar]
- 3.Brandt CM, Spellerberg B. Human infections due to Streptococcus dysgalactiae subspecies equisimilis. Clin Infect Dis 2009;49:766-72. [Google Scholar]
- 4.Erden T, Gültepe BS, Küçükdurmaz F. Periprosthetic joint infection with Streptococcus dysgalactiae subspecies equisimilis: Case report. Jt Dis Relat Surg 2020;31:399-402. [Google Scholar]
- 5.Pham TT, Lazarevic V, Gaia N, Girard M, Cherkaoui A, Suva D, et al. Second periprosthetic joint infection caused by Streptococcus dysgalactiae: How genomic sequencing can help defining the best therapeutic strategy. Front Med (Lausanne) 2020;7:53. [Google Scholar]
- 6.Edwards MS, Baker CJ. Group B streptococcal infections in elderly adults. Clin Infect Dis 2005;41:839-47. [Google Scholar]
- 7.Triffault-Fillit C, Ferry T, Laurent F, Pradat P, Dupieux C, Conrad A, et al. Microbiologic epidemiology depending on time to occurrence of prosthetic joint infection: A prospective cohort study. Clin Microbiol Infect 2019;25:353-8. [Google Scholar]
- 8.Zeller V, Lavigne M, Leclerc P, Lhotellier L, Graff W, Ziza JM, et al. Group B streptococcal prosthetic joint infections: A retrospective study of 30 cases. Presse Med 2009;38:1577-84. [Google Scholar]
- 9.Odum SM, Fehring TK, Lombardi AV, Zmistowski BM, Brown NM, Luna JT, et al. Irrigation and debridement for periprosthetic infections: Does the organism matter? J Arthroplasty 2011;26:114-8. [Google Scholar]
- 10.Argemi X, Hansmann Y, Riegel P, Prévost G. Is Staphylococcus lugdunensis significant in clinical samples? J Clin Microbiol 2017;55:3167-74. [Google Scholar]
- 11.Herry Y, Lesens O, Bourgeois G, Maillet M, Bricca R, Cazorla C, et al. Staphylococcus lugdunensis prosthetic joint infection: A multicentric cohort study. J Infect 2022;85:652-9. [Google Scholar]
- 12.Kunutsor SK, Whitehouse MR, Blom AW, Beswick AD, INFORM Team. Patient-related risk factors for periprosthetic joint infection after total joint arthroplasty: A systematic review and meta-analysis. PLoS One 2016;11:e0150866. [Google Scholar]
- 13.Zhu Y, Zhang F, Chen W, Liu S, Zhang Q, Zhang Y. Risk factors for periprosthetic joint infection after total joint arthroplasty: A systematic review and meta-analysis. J Hosp Infect 2015;89:82-9. [Google Scholar]
- 14.Beam E, Osmon D. Prosthetic joint infection update. Infect Dis Clin North Am 2018;32:843-59. [Google Scholar]
- 15.Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev 2014;27:302-45. [Google Scholar]
- 16.Ikebe T, Murayama S, Saitoh K, Yamai S, Suzuki R, Isobe J, et al. Surveillance of severe invasive group-G streptococcal infections and molecular typing of the isolates in Japan. Epidemiol Infect 2004;132:145-9. [Google Scholar]
- 17.Rajagopal L. Understanding the regulation of Group B Streptococcal virulence factors. Future Microbiol 2009;4:201-21. [Google Scholar]
- 18.Magana-Arachchi DN, Wanigatunge RP. Ubiquitous waterborne pathogens. Waterborne Pathogens 2020;2:15-42. [Google Scholar]
- 19.Barrett L, Atkins B. The clinical presentation of prosthetic joint infection. J Antimicrob Chemother 2014;69:i25-7. [Google Scholar]
- 20.Deacon JM, Pagliaro AJ, Zelicof SB, Horowitz HW. Prophylactic use of antibiotics for procedures after total joint replacement. J Bone Joint Surg Am 1996;78:1755-70. [Google Scholar]
- 21.Li T, Zhang H, Chan PK, Fung WC, Fu H, Chiu KY. Risk factors associated with surgical site infections following joint replacement surgery: A narrative review. Arthroplasty 2022;4:11. [Google Scholar]
- 22.Kane P, Chen C, Post Z, Radcliff K, Orozco F, Ong A. Seasonality of infection rates after total joint arthroplasty. Orthopedics 2014;37:e182-6. [Google Scholar]