Being resistant to chemo and radiotherapy, localized proximal humerus chondrosarcomas could be definitely treated with the MUTARS shoulder hemiarthroplasty system with favorable clinical and functional outcomes.
Dr. Hussein Wehbe, Department of Orthopedics and Traumatology, Lebanese University, Faculty of Medicine, Beirut, Lebanon. E-mail: hssein_wehbe@hotmail.com
Introduction: The incidence of chondrosarcomas is relatively high as it comes second to multiple myeloma as the most common primary malignant bony neoplasms in adults. They tend to occur mostly in the axial skeleton. Hence, they rarely develop in the proximal humerus. Although imaging can aid in the diagnosis of chondrosarcoma, histopathology is the cornerstone that correlates with prognosis and guides us toward the most appropriate treatment modality. Surgical treatment is the best option for chondrosarcomas as most of them are resistant to chemotherapy and radiotherapy. It is really challenging to settle on one surgical technique for proximal humerus chondrosarcomas as surgeons must balance between saving the patient from the oncological process and maintaining a good function of the shoulder joint.
Case Report: We present herein a rare case, the first in Lebanon, of chondrosarcoma hitting the left proximal humerus of a 62-year-old lady successfully managed by operative resection and reconstruction with a cemented shoulder hemiarthroplasty using the Modular Universal Tumor and Revision System (MUTARS®) system.
Conclusion: Chondrosarcomas are relatively rare. Their resistance to chemotherapy and radiation therapy in addition to their proximal humerus localization is troublesome for both the patient and the surgeon. Hence, a relatively new technique (first in Lebanon and the Middle East), the MUTARS shoulder hemiarthroplasty is found to have promising results on terms of morbidity and mortality for the patient when indicated and properly done.
Keywords: Shoulder, chondrosarcomas, hemiarthroplasty, modular universal tumor and revision system.
Bone tumors are tumors invading the bone primarily or secondary due to metastasis. Primary bone tumors can be malignant or benign. Among malignant primary bone tumors, chondrosarcomas, composed of chondrocytes, present with variable degrees of malignancy. According to various studies, it comes third after osteosarcoma and multiple myeloma as the most common primary malignant bone tumors in all age groups. However, being a tumor of adulthood, chondrosarcoma is the second most common primary malignant bone tumor after multiple myeloma [1, 2]. As presentation, they most commonly affect the axial skeleton (pelvis, sternum, scapula) then the proximal femur and proximal humerus. In the case of chondrosarcoma, the degree of malignancy is not always correlated with histology since sometimes chondrosarcomas with similar histological descriptions could be benign in the hand but malignant if affecting the long bones. Hence, tumor location is an important factor in tumor aggressiveness. Chondrosarcomas are typically found in older patients (40–75 years) with slight male predominance. They are divided based on histopathology into two types: Conventional or primary type (80–90%) and non-conventional or secondary forms arising from benign cartilage lesions as osteochondromas or enchondromas. These benign tumors can be solitary or may present with multiple lesions, which are more common, as in cases of Maffucci syndrome or Ollier’s disease [3,4]. Furthermore, chondrosarcomas can be subdivided based on a particular histological grading system which determines the prognosis and guides the treatment. Most of them are low grade (1 or 2) and characterized by a similar histological appearance to enchondromas. Grade 3 is dedifferentiated chondrosarcomas developing initially from low-grade chondroid lesions to high-grade neoplasm (spindle cells, high nuclear atypia, and rare cartilaginous matrix on histology) [5,6]. In addition to dedifferentiated chondrosarcomas, two more subtypes of primary chondrosarcomas are clear cell chondrosarcoma and mesenchymal chondrosarcoma. Clinical presentation varies according to tumor grade and location. Pain, redness, and edema are the main signs and symptoms when predominantly involving long bones but it can also present with gastrointestinal (bowel obstruction) or genitourinary (bladder obstruction) symptoms if affecting the pelvic bones or with a pathologic fracture in high-grade tumors. Radiographs are the first step in establishing the diagnosis. It helps identifying whether it is a lytic or blastic lesion, low-grade or high-grade chondrosarcomas. For more illustration, low-grade chondrosarcomas appear as lucent medullary lesions with intralesional calcifications (popcorn pattern), cortical thickening, and endosteal erosions. On the other hand, destroyed cortex and soft-tissue mass expansion are seen in high-grade lesions. Magnetic resonance imaging (MRI) is useful for comprehensive evaluation of primary tumor and possible marrow and soft-tissue involvement. Detection of cortical involvement, matrix calcification, and deep endosteal scalloping in low-grade neoplasms is best accomplished by computed tomography. In general, early diagnosis of chondrosarcoma is associated with a favorable prognosis and histopathology remains the cornerstone that guides us to the most appropriate treatment modality. The management of chondrosarcomas is mainly operative either by intralesional curettage or wide surgical excision. Curettage with adjuvant treatment is reserved for central low-grade tumors located in the extremities (minimal rate of metastasis) [7,8]. Indications for wide surgical excision include intermediate and high-grade chondrosarcomas, low-grade lesions in the axial skeleton, and cases of soft tissue or joint involvement [9,10]. Chemotherapy and radiation therapy are known to be less effective for most chondrosarcomas. Chemotherapy use in dedifferentiated chondrosarcomas is controversial. However, it is useful as neoadjuvant for mesenchymal chondrosarcomas [11]. Radiotherapy can be beneficial in cases of unresectable lesions or after intralesional curettage as adjuvant therapy [12]. Histological grade correlates with survival. About 90% of Grade 1, 60–70% of Grade 2, 30–50% of Grade 3, and <10% of de-differentiated chondrosarcomas have a 5-year survival rate.
A 62-year-old female patient presented to our orthopedic clinic at Bahman University Hospital in Beirut, Lebanon, for pain and minimal limitation in the range of motion in her left shoulder for several months. No history of trauma was reported. She is known to have a type 2 diabetes mellitus. Her family history revealed that her father had a “non-bony” cancer. Pain is at rest exacerbated by passive flexion and abduction. Her pain was well controlled by non-steroidal anti-inflammatory drugs but then became more severe and resistant. On inspection, there was no redness, but mild swelling was inspected. Palpation showed mild tenderness over the proximal humerus. No decrease in the passive range of motion but limitations in the active range of motion were noted. The range of motion was 0–90° of abduction, 0–100° of flexion and external rotation at side 0–30°, internal rotation to T9-T10 vertebral height. Neurovascular examination is intact. There were no major abnormal laboratory findings.
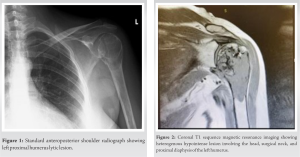
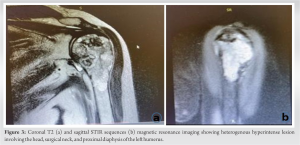
As the next step, X-rays of the left shoulder were done and revealed a lytic lesion with endosteal scalloping and cortical changes (Fig. 1). To assess soft-tissue involvement, MRI of the left shoulder was performed which showed a heterogenous expensile mass mainly hypointense on T1 (Fig. 2) and hyperintense on T2 (Fig. 3a) and STIR (Fig. 3b) with chondroid matrix involving head, surgical neck, and proximal diaphysis of the left humerus measuring 82 × 43 mm. The mass showed periosteal thickening and extraosseous extension. There was no extension into glenohumeral or acromioclavicular joint but there was mild sub-acromial effusion. Moreover, rotator cuff tendons were intact with no extension.
For metastatic assessment, positron emission tomography scan was performed. No abnormal findings were seen beside the lytic hypermetabolic lesion involving the humeral head, metaphysis, and proximal diaphysis of the left humerus. For definitive diagnosis, an incisional biopsy was done which showed cartilaginous tumor growing into lobules of varying sizes. Chondrocytes were double nucleated, moderately enlarged, hyperchromatic, rarely mitotic, and located within lacunae. Furthermore, the matrix was solid and pale blue. Hence, a diagnosis of left proximal humerus chondrosarcoma was confirmed with findings correlated with a Grade 1 lesion. Unfortunately, the patient had difficult circumstances that forced her to procrastinate during the management plan. Thus, the MRI was repeated 5 months later for pre-operative planning and comprehensive evaluation of tumor progression. It revealed a mild increase in tumor size to 85 × 48 mm with no other significant changes. The patient was planned for a limb salvage procedure with wide surgical excision and reconstruction.
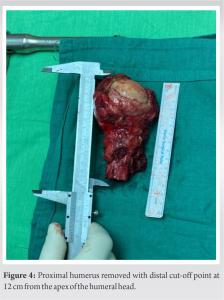
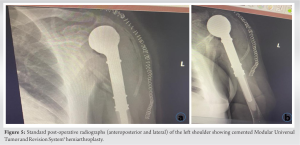
Intraoperatively, the patient was put in a beach chair position with a support arm holder and under general anesthesia. An extended deltopectoral approach was adopted. A careful dissection of the subcutaneous tissue with retraction of the cephalic vein was performed. Opening of the deltopectoral interval and incision with dissection of the clavipectoral fascia just lateral to the conjoint tendon was followed meticulously to avoid musculocutaneous nerve injury until reaching the bone. Osteotomy of the proximal humerus 12-cm distal from the apex was performed (Fig. 4). Then, reconstruction of the proximal humerus was done with a cemented shoulder hemiarthroplasty using Modular Universal Tumor and Revision System (MUTARS®), a single design implant system coated with titanium. After the final implant assembly, the prosthesis was covered by a polyethylene tube which was attached to the joint capsule, then tightened and fixed over and under the pads of the prosthesis. The rotator cuff tendons were fixed with sutures to the meshes of the tube proximally at the level of the implant metaphysis which acts as the greater and lesser humeral tuberosities, whereas the mesh around the diaphysis was the site of reattachment of the deltoid and pectoralis major muscles. Lavage and hemostasis were performed. Three layers of closure and a closed suction drainage were attached. Finally, the shoulder was put in a sling immobilizer to assist in shoulder stability and recovery. Post-operative X-rays were taken at the same day (Fig. 5).
The patient had a smooth post-operative course with hospital discharge at day 5 after surgery. During her hospital stay, physical therapy with passive shoulder immobilization was initiated. Moreover, 20 sessions of physiotherapy were done after discharge. There is no chemo or radiotherapy received. Histopathology showed a cartilaginous proliferation with focal myxoid changes, moderate cellularity, mild-to-moderate nuclear atypia, and rare mitosis. There was no necrosis seen. It also showed that the tumor infiltrates the periosteal soft tissue at the level of the head of the humerus. However, the surgical margin was free with a distal surgical margin at 4 cm from the tumor. Immunohistochemical study reveals diffuse positivity of the tumor cells for S100 with low expression of Ki67. Hence, the diagnosis after resection showed a moderately differentiated Grade 2 chondrosarcoma measuring 9.5 cm. After 3 months of follow-up, the functional results were acceptable showing no pain and active abduction limitation to 90° with no passive limitation in range of motion. After 1 year, the patient is doing very well with active abduction reaching 110–115°, forward flexion 120°, 10–15° external rotation, and internal rotation reaching T7-T8 vertebral height.
Chondrosarcomas, whether low-grade or high-grade tumors, are a heterogenous type of bone neoplasms classified based on histology [11]. Being heterogenous, they have various morphological characteristics depending on if they are conventional or non-conventional [13]. Moreover, the clinical behavior of this pathology varies according to the location of the disease and histology. Thus, a chondrosarcoma in a hand can be of indolent form while a long bone tumor will be more aggressive. Chondrosarcomas are most commonly found in the axial skeleton. Proximal humerus chondrosarcomas localization is relatively a rare entity of this heterogenous group [14]. The primary approach to this kind of bone neoplasm can be made by imaging alone although biopsy is the diagnostic modality of choice if uncertainty occurs. A coordinated multidisciplinary approach is necessary to manage patients with chondrosarcomas since most are resistant to chemotherapy and radiation therapy [11]. Hence, surgery is the treatment modality of choice [15-17]. In the past, surgeons had limited choices for treating proximal humerus tumors and they lastly resorted to a scapulohumeral disarticulation for such tumors. However, the advancements in surgical and biomedical engineering fields provided orthopedists with better surgical options regarding bone tumor resection and subsequent limb reconstruction. Furthermore, the evolution of various diagnostic techniques helped in the diagnosis of the tumor in an earlier stage that can be more adequately managed along with saving the limb. Therefore, a disarticulation procedure is rare nowadays and reserved for cases with brachial plexus or axillary involvement [18-20]. New reconstruction techniques have been developed to decrease as much as possible the probability of future recurrences and preserve the shape and function of the limb allowing the patient to stay involved in his/her daily activities [21,22]. It usually involves wide surgical resection with negative margins but with the least possible to preserve functionality. There is no specific best reconstruction technique for all patients. Each patient is a unique case that needs a specific technique according to his/her age, comorbidities, life expectancy, lifestyle, and the need to preserve functionality [23,24]. Recent advances in orthopedic oncology allowed proximal humerus reconstruction through various techniques: autografts, allografts, implanted prostheses, or prosthetic-biological composites [22,24-28]. Younger patients with long life expectancy are most suited for an allograft as its functionality improves with time and successful allograft reconstruction has a low probability for a revision surgery even after decades [14,29,30], whereas patients who receive chemotherapy or radiotherapy and/or have healing defects (diabetes, advanced age, neurovascular, or metabolic disorders) may have high complication rates (chondrolysis, graft failure, subchondral collapse, and implant fracture) if they undergo allograft reconstruction [22,24-28,30-34]. On the other hand, autograft is best indicated for pediatric patients and young patients with long life expectancy and good bone quality at the donor site as it has the best osteointegrative potential. Growing plates can be used in children to assist the development of the shoulder. However, this technique often requires long surgical duration, an increasing amount of graft harvest, and thus donor site morbidity [35-41]. Moreover, anatomical implanted prostheses are indicated in advanced-age or middle-aged patients with damage to the deltoid muscle or axillary nerve. This modality has less surgical time and demand than the previous modalities and it is associated with earlier functional recovery; however, it has a limited durability and restricted functional outcome [42-49]. Furthermore, reverse shoulder prostheses are preserved for middle-aged patients having maintained deltoid insertion and axillary nerve and request high functional abilities. This type of prostheses has also a limited durability but it has a better mobility than the anatomical endoprostheses [42,50-55]. Prosthetic biological composites are indicated for both young mid-age patients with high functional demands and medium or long life expectancy. This modality is a long complex procedure with relatively high mechanical complications but with subsequent fair durability and satisfactory functional outcomes [56-61]. Nevertheless, biological treatment compared to endoprostheses and allograft-prosthesis composite had more complications thus necessitating more subsequent re-interventions [26]. Despite there is no best reconstruction method, reverse shoulder arthroplasty yields the most satisfactory functional outcomes [45,53,62]. The primary objective for all the aforementioned techniques is to restore shoulder stability and mobility. Chondrosarcomas necessitate challenging multidisciplinary management. An approach balancing between an extensive tumor resection respecting the oncological principles and a limb-sparing procedure preserving the complex shoulder anatomy and its functionality creates a huge burden on the surgeon. In our case, we took into consideration its particularities and the principles of each technique to decide on the best treatment modality that can provide the patient with acceptable shoulder stability and functionality. The tumor was not extending into the glenohumeral or acromioclavicular joints, the patient is <70 years of age, and she was able to achieve forward flexion more than 90°; thus, we decided to go for hemiarthroplasty. The tumor, according to the last MRI 2 months preoperatively, was 85 mm in length into the humeral diaphysis, and thus, an extensive resection of the proximal humerus and humeral shaft was required. Thereby, we needed a long-stem tumoral prosthesis able to restore length, stability, and motion. Hence, the decision was made on a cement MUTARS® titanium-coated endoprosthesis which was developed to treat major osseous defects in the extremities. After careful dissection and a thorough assessment of the extent of tumor invasion, no surrounding soft tissue or joint space involvement was seen. Thus, a Malawer type 1 intra-articular proximal humerus resection at 12 cm from the apex of the humeral head with a maximal preservation of the surrounding soft tissue was performed. To prepare the medullary canal, progressive rasping was performed to a size of 12 mm. We applied the cement within the medullary cavity, then we mounted and impacted a 10 mm in diameter and 160 mm in length original titanium coated MUTARS® stem. After cement hardening, proximal components were assembled with the appropriate rotation. A trial cap was used to control muscle tension and then removed after sufficient tension was achieved. Around 20° of implant retroversion was applied and the final MUTARS® components were assembled. Finally, an attachment polyethylene terephthalate tube was packed around the implant. Its role is to facilitate reattachment of the joint capsule and the remaining tendinous insertion (rotator cuff, deltoid, pectoralis major) to the prosthesis. The tube was then tightened and fixed under the pads of the MUTARS® components. Finally, muscles and tendons were fixed with non-resorbable sutures to the meshes of the tube. The histopathological examination postoperatively showed an evolved grading of the disease from Grade 1 at the time of biopsy to Grade 2 moderately differentiated chondrosarcoma measuring 9.5 cm with negative resection margins. In addition to the localization and since the progression of the tumor grade and size was relatively slow, the oncological outcome is favorable, and humeral reconstruction is expected to last as predicted. The post-operative course was uneventful with no major complications. No chemotherapy or radiotherapy was needed. Moreover, she received progressive functional rehabilitation with remarkable improvement in shoulder function (passive abduction and forward flexion to 90°) and pain score. Recent follow-up revealed satisfactory functional status and no disease recurrence. Continuous follow-up is being done. Despite the 7-month delay between the diagnosis and surgical treatment which resulted in an increase in tumor grade and size, we were able to obtain an acceptable functional and pain-free outcome in the first 3 months after surgery. The MUTARS® system proved in our case that it can help patients requiring extensive proximal humeral resection to regain their shoulder joint functionality in a short period of time. Note that this case is the first reported one in Lebanon and the Middle East. Furthermore, the literature showed limited studies on the outcome of the MUTARS® system reconstruction for proximal humerus chondrosarcomas. Therefore, our case management and outcomes could encourage the use of this system in similar cases in the future for better outcomes clinically and functionally and to increase life expectancy.
Proximal humerus localization for chondrosarcoma is rare and its management is challenging. Surgery is the best resort for definitive treatment since frequent resistance to chemotherapy and radiotherapy is found with most subtypes. Nowadays, reconstruction techniques have been proved to give promising outcomes. Hence, the MUTARS® system, developed to replace major osseous defects, showed in our case favorable outcomes. However, further comparative studies should be conducted to prove the efficacy of this system relative to other treatment options to support the use of the MUTARS system in similar cases in the ultimate interest of patient benefit and satisfaction.
Localized symptomatic large proximal humerus chondrosarcomas could be definitely cured surgically by excising the large defects and implanting a MUTARS shoulder hemiarthroplasty system that showed in our case promising functional and clinical outcomes.
References
- 1.Rozeman LB, Cleton-Jansen AM, Hogendoorn PC. Pathology of primary malignant bone and cartilage tumours. Int Orthop 2006;30:437-44. [Google Scholar]
- 2.Van Praag Veroniek VM, Rueten-Budde AJ, Ho V, Dijkstra PD, Study Group Bone and Soft Tissue Tumours (WeBot), Fiocco M, et al. Incidence, outcomes and prognostic factors during 25 years of treatment of chondrosarcomas. Surg Oncol 2018;27:402-8. [Google Scholar]
- 3.Verdegaal SH, Bovée JV, Pansuriya TC, Grimer RJ, Ozger H, Jutte PC, et al. Incidence, predictive factors, and prognosis of chondrosarcoma in patients with Ollier disease and Maffucci syndrome: An international multicenter study of 161 patients. Oncologist 2011;16:1771-9. [Google Scholar]
- 4.Ryzewicz M, Manaster BJ, Naar E, Lindeque B. Low-grade cartilage tumors: Diagnosis and treatment. Orthopedics 2007;30:35-46. [Google Scholar]
- 5.Rosenthal DI, Schiller AL, Mankin HJ. Chondrosarcoma: Correlation of radiological and histological grade. Radiology 1984;150:21-6. [Google Scholar]
- 6.Ene R, Sinescu RD, Ene P, Popescu D, Cîrstoiu MM, Cîrstoiu FC. Proximal tibial osteosarcoma in young patients: Early diagnosis, modular reconstruction. Rom J Morphol Embryol 2015;56:413-7. [Google Scholar]
- 7.Hanna SA, Whittingham-Jones P, Sewell MD, Pollock RC, Skinner JA, Saifuddin A, et al. Outcome of intralesional curettage for low-grade chondrosarcoma of long bones. Eur J Surg Oncol 2009;35:1343-7. [Google Scholar]
- 8.Chen X, Yu LJ, Peng HM, Jiang C, Ye CH, Zhu SB, et al. Is intralesional resection suitable for central grade 1 chondrosarcoma: A systematic review and updated meta-analysis. Eur J Surg Oncol 2017;43:1718-26. [Google Scholar]
- 9.Leddy LR, Holmes RE. Chondrosarcoma of bone. Cancer Treat Res 2014;162:117-30. [Google Scholar]
- 10.Dai X, Ma W, He X, Jha RK. Review of therapeutic strategies for osteosarcoma, chondrosarcoma, and Ewing’s sarcoma. Med Sci Monit 2011;17:A177-90. [Google Scholar]
- 11.Riedel RF, Larrier N, Dodd L, Kirsch D, Martinez S, Brigman BE. The clinical management of chondrosarcoma. Curr Treat Options Oncol 2009;10:94-106. [Google Scholar]
- 12.Moussavi-Harami F, Mollano A, Martin JA, Ayoob A, Domann FE, Gitelis S, et al. Intrinsic radiation resistance in human chondrosarcoma cells. Biochem Biophys Res Commun 2006;346:379-85. [Google Scholar]
- 13.Thorkildsen J, Taksdal I, Bjerkehagen B, Haugland HK, Børge Johannesen T, Viset T, et al. Chondrosarcoma in Norway 1990-2013; An epidemiological and prognostic observational study of a complete national cohort. Acta Oncol 2019;58:273-82. [Google Scholar]
- 14.Mourikis A, Mankin HJ, Hornicek FJ, Raskin KA. Treatment of proximal humeral chondrosarcoma with resection and allograft. J Shoulder Elbow Surg 2007;16:519-24. [Google Scholar]
- 15.Shen ZN, Nishida K, Doi H, Oohashi T, Hirohata S, Ozaki T, et al. Suppression of chondrosarcoma cells by 15-deoxy-Delta 12,14-prostaglandin J2 is associated with altered expression of Bax/Bcl-xL and p21. Biochem Biophys Res Commun 2005;328:375-82. [Google Scholar]
- 16.Kim DW, Seo SW, Cho SK, Chang SS, Lee HW, Lee SE, et al. Targeting of cell survival genes using small interfering RNAs (siRNAs) enhances radiosensitivity of Grade II chondrosarcoma cells. J Orthop Res 2007;25:820-8. [Google Scholar]
- 17.Kim DW, Kim KO, Shin MJ, Ha JH, Seo SW, Yang J, et al. siRNA-based targeting of antiapoptotic genes can reverse chemoresistance in P-glycoprotein expressing chondrosarcoma cells. Mol Cancer 2009;8:28. [Google Scholar]
- 18.Gitelis S, Bertoni F, Picci P, Campanacci M. Chondrosarcoma of bone. The experience at the Istituto Ortopedico Rizzoli. J Bone Joint Surg Am 1981;63:1248-57. [Google Scholar]
- 19.Leerapun T, Hugate RR, Inwards CY, Scully SP, Sim FH. Surgical management of conventional grade I chondrosarcoma of long bones. Clin Orthop Relat Res 2007;463:166-72. [Google Scholar]
- 20.Bindiganavile S, Han I, Yun JY, Kim HS. Long-term outcome of chondrosarcoma: A single institutional experience. Cancer Res Treat 2015;47:897-903. [Google Scholar]
- 21.Bruns J, Elbracht M, Niggemeyer O. Chondrosarcoma of bone: An oncological and functional follow-up study. Ann Oncol 2001;12:859-64. [Google Scholar]
- 22.Malawer MM, Sugarbaker PH, Lampert M, Baker AR, Gerber NL. The Tikhoff-Linberg procedure: Report of ten patients and presentation of a modified technique for tumors of the proximal humerus. Surgery 1985;97:518-28. [Google Scholar]
- 23.Yang Q, Li J, Yang Z, Li X, Li Z. Limb sparing surgery for bone tumours of the shoulder girdle: The oncological and functional results. Int Orthop 2010;34:869-75. [Google Scholar]
- 24.Kassab M, Dumaine V, Babinet A, Ouaknine M, Tomeno B, Anract P. Reconstructions after tumoral resection of the proximal humerus: An average of 7 years retrospective series of 29 reconstructions. Rev Chir Orthop Réparatrice Appar Mot 2005;91:15-23. [Google Scholar]
- 25.Rödl RW, Gosheger G, Gebert C, Lindner N, Ozaki T, Winkelmann W. Reconstruction of the proximal humerus after wide resection of tumours. J Bone Joint Surg Br 2002;84:1004-8. [Google Scholar]
- 26.Asavamongkolkul A, Eckardt JJ, Eilber FR, Dorey FJ, Ward WG, Kelly CM, et al. Endoprosthetic reconstruction for malignant upper extremity tumors. Clin Orthop Relat Res 1999;360:207-20. [Google Scholar]
- 27.Van de Sande MA, Dijkstra PD, Taminiau AH. Proximal humerus reconstruction after tumour resection: Biological versus endoprosthetic reconstruction. Int Orthop 2011;35:1375-80. [Google Scholar]
- 28.Abdeen A, Hoang BH, Athanasian EA, Morris CD, Boland PJ, Healey JH. Allograft-prosthesis composite reconstruction of the proximal part of the humerus: Functional outcome and survivorship. J Bone Joint Surg Am 2009;91:2406-15. [Google Scholar]
- 29.Getty PJ, Peabody TD. Complications and functional outcomes of reconstruction with an osteoarticular allograft after intra-articular resection of the proximal aspect of the humerus. J Bone Joint Surg Am 1999;81:1138-46. [Google Scholar]
- 30.Horowitz RA, Leventis MD, Rohrer MD, Prasad HS. Bone grafting: History, rationale, and selection of materials and techniques. Compend Contin Educ Dent 2014;35:1-6. [Google Scholar]
- 31.Aponte-Tinao LA, Ayerza MA, Muscolo DL, Farfalli GL. Allograft reconstruction for the treatment of musculoskeletal tumors of the upper extremity. Sarcoma 2013;2013:925413. [Google Scholar]
- 32.Gebhardt MC, Roth YF, Mankin HJ. Osteoarticular allografts for reconstruction in the proximal part of the humerus after excision of a musculoskeletal tumor. J Bone Joint Surg Am 1990;72:334-45. [Google Scholar]
- 33.DeGroot H, Donati D, Di Liddo M, Gozzi E, Mercuri M. The use of cement in osteoarticular allografts for proximal humeral bone tumors. Clin Orthop Relat Res 2004;427:190-7. [Google Scholar]
- 34.Yao W, Cai Q, Wang J, Hou J. Mid-to long-term effects of two different biological reconstruction techniques for the treatment of humerus osteosarcoma involving caput humeri. World J Surg Oncol 2020;18:23. [Google Scholar]
- 35.Kumar VP, Satku K. Osteoarticular allografts for reconstruction in the proximal part of the humerus after excision of a musculoskeletal tumor. J Bone Joint Surg Am 1992;74:152. [Google Scholar]
- 36.Wada T, Usui M, Isu K, Yamawakii S, Ishii S. Reconstruction and limb salvage after resection for malignant bone tumour of the proximal humerus. A sling procedure using a free vascularised fibular graft. J Bone Joint Surg Br 1999;81:808-13. [Google Scholar]
- 37.Liu T, Zhang Q, Guo X, Zhang X, Li Z, Li X. Treatment and outcome of malignant bone tumors of the proximal humerus: Biological versus endoprosthetic reconstruction. BMC Musculoskelet Disord 2014;15:69. [Google Scholar]
- 38.Landau MJ, Badash I, Yin C, Alluri RK, Patel KM. Free vascularized fibula grafting in the operative treatment of malignant bone tumors of the upper extremity: A systematic review of outcomes and complications. J Surg Oncol 2018;117:1432-9. [Google Scholar]
- 39.Tsukushi S, Nishida Y, Takahashi M, Ishiguro N. Clavicula pro humero reconstruction after wide resection of the proximal humerus. Clin Orthop Relat Res 2006;447:132-7. [Google Scholar]
- 40.Zekry KM, Yamamoto N, Hayashi K, Takeuchi A, Alkhooly AZ, Abd-Elfattah AS, et al. Reconstruction of intercalary bone defect after resection of malignant bone tumor. J Orthop Surg (Hong Kong) 2019;27(1):2309499019832970. [Google Scholar]
- 41.Steiger CN, Journeau P, Lascombes P. The role of the periosteal sleeve in the reconstruction of bone defects using a non-vascularised fibula graft in the pediatric population. Orthop Traumatol Surg Res 2017;103:1115-20. [Google Scholar]
- 42.Manfrini M, Tiwari A, Ham J, Colangeli M, Mercuri M. Evolution of surgical treatment for sarcomas of proximal humerus in children: Retrospective review at a single institute over 30 years. J Pediatr Orthop 2011;31:56-64. [Google Scholar]
- 43.Gupta GR, Yasko AW, Lewis VO, Cannon CP, Raymond AK, Patel S, et al. Risk of local recurrence after deltoid-sparing resection for osteosarcoma of the proximal humerus. Cancer 2009;115:3767-73. [Google Scholar]
- 44.Wittig JC, Bickels J, Kellar-Graney KL, Kim FH, Malawer MM. Osteosarcoma of the proximal humerus: Long-term results with limb-sparing surgery. Clin Orthop Relat Res 2002;397:156-76. [Google Scholar]
- 45.Mayilvahanan N, Paraskumar M, Sivaseelam A, Natarajan S. Custom mega-prosthetic replacement for proximal humeral tumours. Int Orthop 2006;30:158-62. [Google Scholar]
- 46.Streitbuerger A, Henrichs M, Gosheger G, Ahrens H, Nottrott M, Guder W, et al. Improvement of the shoulder function after large segment resection of the proximal humerus with the use of an inverse tumour prosthesis. Int Orthop 2015;39:355-61. [Google Scholar]
- 47.Gosheger G, Gebert C, Ahrens H, Streitbuerger A, Winkelmann W, Hardes J. Endoprosthetic reconstruction in 250 patients with sarcoma. Clin Orthop Relat Res 2006;450:164-71. [Google Scholar]
- 48.Raiss P, Kinkel S, Sauter U, Bruckner T, Lehner B. Replacement of the proximal humerus with MUTARS tumor endoprostheses. Eur J Surg Oncol 2010;36:371-7. [Google Scholar]
- 49.Kumar D, Grimer RJ, Abudu A, Carter SR, Tillman RM. Endoprosthetic replacement of the proximal humerus. J Bone Joint Surg Br 2003;85:717-22. [Google Scholar]
- 50.Ross AC, Wilson JN, Scales JT. Endoprosthetic replacement of the proximal humerus. J Bone Joint Surg Br 1987;69:656-61. [Google Scholar]
- 51.De Wilde LF, Plasschaert FS, Audenaert EA, Verdonk RC. Functional recovery after a reverse prosthesis for reconstruction of the proximal humerus in tumor surgery. Clin Orthop Relat Res 2005;430:156-62. [Google Scholar]
- 52.Bonnevialle N, Mansat P, Lebon J, Laffosse JM, Bonnevialle P. Reverse shoulder arthroplasty for malignant tumors of proximal humerus. J Shoulder Elbow Surg 2015;24:36-44. [Google Scholar]
- 53.Guven MF, Aslan L, Botanlioglu H, Kaynak G, Kesmezacar H, Babacan M. Functional outcome of reverse shoulder tumor prosthesis in the treatment of proximal humerus tumors. J Shoulder Elbow Surg 2016;25:e1-6. [Google Scholar]
- 54.De Wilde L, Boileau P, Van der Bracht H. Does reverse shoulder arthroplasty for tumors of the proximal humerus reduce impairment? Clin Orthop Relat Res 2011;469:2489-95. [Google Scholar]
- 55.Ackland DC, Roshan-Zamir S, Richardson M, Pandy MG. Moment arms of the shoulder musculature after reverse total shoulder arthroplasty. J Bone Joint Surg Am 2010;92:1221-30. [Google Scholar]
- 56.Ji T, Tang X, Guo W. Enhancing soft-tissue reattachment in proximal humeral endoprosthetic reconstruction. J Orthop Surg (Hong Kong) 2014;22:100-3. [Google Scholar]
- 57.Abdeen A, Healey JH. Allograft-prosthesis composite reconstruction of the proximal part of the humerus: Surgical technique. J Bone Joint Surg Am 2010;92 Suppl 1 Pt 2:188-96. [Google Scholar]
- 58.Moran M, Stalley PD. Reconstruction of the proximal humerus with a composite of extracorporeally irradiated bone and endoprosthesis following excision of high grade primary bone sarcomas. Arch Orthop Trauma Surg 2009;129:1339-45. [Google Scholar]
- 59.Lozano-Calderón SA, Chen N. Proximal humerus allograft prosthetic composites: Technique, outcomes, and pearls and pitfalls. Curr Rev Musculoskelet Med 2015;8:324-33. [Google Scholar]
- 60.King JJ, Nystrom LM, Reimer NB, Gibbs CP Jr., Scarborough MT, Wright TW. Allograft-prosthetic composite reverse total shoulder arthroplasty for reconstruction of proximal humerus tumor resections. J Shoulder Elbow Surg 2016;25:45-54. [Google Scholar]
- 61.El Beaino M, Liu J, Lewis VO, Lin PP. Do early results of proximal humeral allograft-prosthetic composite reconstructions persist at 5-year follow up? Clin Orthop Relat Res 2019;477:758-65. [Google Scholar]
- 62.Black AW, Szabo RM, Titelman RM. Treatment of malignant tumors of the proximal humerus with allograft-prosthesis composite reconstruction. J Shoulder Elbow Surg 2007;16:525-33. [Google Scholar]