Diagnosing giant cell tumors in achondroplasia patients is challenging due to overlapping histopathology and altered radiological features post-denosumab treatment, requiring careful evaluation for accurate management.
Dr. Rajesh Rana, Department of orthopedics, SCB Medical college & Hospital, Cuttack, Odisha, India. E-mail: rajesh.rana66@gmail.com
Introduction: Giant cell tumor of bone (GCTB) is a rare yet locally aggressive neoplasm primarily affecting young adults. Its hallmark features include multinucleated osteoclast-type giant cells and mononuclear tumor cells. Treatment with denosumab, an anti-RANK ligand antibody, has shown efficacy, but it alters histomorphology, posing diagnostic challenges. Co-occurrence with achondroplasia, though rare, warrants consideration in bone lesion evaluations.
Case Report: A 30-year-old male with achondroplasia presented with a proximal femur GCT, a rare association. Neoadjuvant denosumab was administered due to thin tumor cortex and cortical breech. Surgical excision with bone cement filling and Philo’s plate supplementation was performed. Histopathological examination post-treatment revealed the absence of osteoclast-type giant cells, extensive necrosis, hyalinization, and mononuclear infiltrates.
Discussion: Denosumab induces a reduction in osteoclast numbers, causing tumor shrinkage and sclerosis, while altering typical GCT histology. Similar findings were noted in the literature, including stromal changes like spindle-shaped cells, inflammation, vascular proliferation, and hemosiderin-laden foamy macrophages. Recognition of these alterations is crucial for accurate diagnosis.
Conclusion: GCT, though rare, presents distinct histopathological features aiding diagnosis. Denosumab treatment modifies tumor morphology, necessitating thorough clinical evaluation for accurate diagnosis post-treatment. Understanding, these challenges is essential for optimal management of GCT cases.
Keywords: Giant cell tumor, achondroplasia, denosumab therapy, challenges in histopathology.
Giant cell tumor of bone (GCTB) is a relatively rare (incidence of about 1.7 in 1 million people per year) [1], benign, but locally aggressive osteolytic skeletal neoplasm of young adults (3rd through 5th decades of life) [2] and are more common in females (female-to-male ratio being about 1.5–1). First described by Cooper and Travers in 1818, it was not until 1940 that GCT was formally distinguished from other benign tumors of bone. It is characterized by the presence of numerous multinucleated osteoclast-type giant cells, hence referred to as “osteoclastoma.” Despite the fact that it is regarded as a benign tumor, GCT can be locally aggressive, can recur locally, and does show occasional metastasis [2], most often to the lungs. It most commonly originates in the epiphyseal-metaphyseal region of mature long bones, the commonest sites being the distal femur, proximal tibia, and distal radius. Proximal femur GCT is a rare presentation. Histologically, there are uniform oval mononuclear tumor cells and abundant osteoclast-type giant cells [3]. The neoplastic cells (primitive osteoblast precursors) have acquired mutations in the H3F3A gene which codes for histone 3.3. These cells also express RANK ligand (RANKL), which promotes the proliferation and differentiation of osteoclast precursors into mature osteoclasts. The expression of RANKL has led to the use of anti-RANKL antibody, denosumab in the treatment of recurrent and surgically unresectable GCT [4, 5]. Although it is considered an effective component in the management of GCT, denosumab causes significant changes in the histomorphology of the tumor which in turn presents a challenge in diagnosis of cases post-treatment. Close interdisciplinary collaboration with a thorough clinical history and work-up thus become essential for accurate diagnosis and optimal management of patients. Achondroplasia is a genetic disorder characterized by abnormal bone growth, resulting in dwarfism. It is the most common form of short-limbed dwarfism, affecting approximately one in 20,000–30,000 live births worldwide. This condition is caused by mutations in the FGFR3 gene, which disrupt normal bone development and growth [6]. Individuals with achondroplasia typically have short stature, disproportionately short limbs, a prominent forehead, and characteristic facial features. Although the association between achondroplasia and GCT is rare, clinicians should be aware of this potential co-occurrence, particularly when evaluating bone lesions in individuals with achondroplasia. Similarly, GCT of the proximal femur is a rare presentation. Here, we present a case report showing proximal femur GCT in an achondroplasia patient.
A 30-year-old male patient presented to the hospital with pain in the left hip and an inability to walk following a trivial fall. The patient was a known case of achondroplasia. On examination, the right proximal thigh was swollen, red, and warm. The proximal femur trochanter was tender, and the range of movement of the left hip was painful. Plain radiographs revealed an expansile lytic lesion in the proximal femur greater trochanter area with a pathological fracture (Fig. 1).
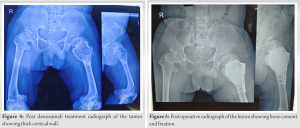
Further evaluation with a computed tomography (CT) scan showed thinned-out cortex with a breach in the cortex, and the lesion appeared osteolytic (Fig. 2). The magnetic resonance imaging revealed the same osteolytic lesion of the proximal femur with a pathological fracture, but there was no soft-tissue extension (Fig. 3). An high-resolution CT of the thorax was performed, and no pulmonary metastasis was found. The lesion was planned for biopsy and histopathological evaluation for a final diagnosis. A needle biopsy was performed, and histopathological evaluation showed the lesion to be a GCT. Since the cortex of the lesion was thin with a breach, neoadjuvant denosumab chemotherapy was
planned.
Six doses of denosumab were given, leading to the formation of a thick cortical wall around the tumor (Fig. 4). The tumor was then removed with extended curettage and filled with bone cement. Supplementary fixation was provided with a Philos plate due to small-sized achondroplastic bones (Fig. 5).
Conventional proximal femur fixation implants were not appropriate for the patient. The curated tissues were sent for histopathological examination.
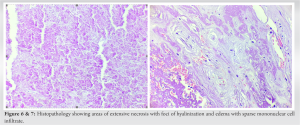
Histopathological changes observed in the GCT after denosumab treatment were quite different from those seen in conventional GCTs (Figs. 6-9). Contrast to the hallmark of GCTB which features abundant osteoclast-type giant cells and mononuclear tumor cells, there was a complete absence of the former in the excised tumor. There were areas of extensive necrosis (Fig. 6) with foci of hyalinization and edema with sparse mononuclear cell infiltrate (Fig. 7).
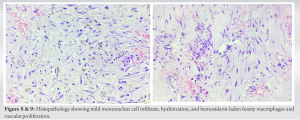
Fig. 8 and 9 show mild mononuclear cell infiltrate, hyalinization, and hemosiderin-laden foamy macrophages and vascular proliferation. The patient is planned for follow-up every 3 months for the first 2 years, then every 6 months for 5 years. At present, the patient has been followed up for 6 months without any recurrence.
The effect of denosumab on the histological appearance of GCT is profound and multifaceted. Although effective in inducing a reduction in osteoclast numbers, leading to tumor shrinkage and sclerosis, it simultaneously alters the typical histological features associated with GCT. The case presented here shows a significant reduction in the number of osteoclast-type giant cells with areas of necrosis, hyalinization, and mononuclear infiltrates. Similar findings were recorded by Kumar et al. [7], where they analyzed clinical records and slides of 11 patients of GCT (four males and seven females) with a mean age of 30 years, who had been administered neoadjuvant denosumab therapy. The pre- and post-therapy GCT specimens were compared where seven out of the 11 cases showed the former findings. Stromal component of the tumor showed significant changes as well. There were abundant spindle-shaped cells arranged in trabeculae, inflammation, vascular proliferation, and the presence of hemosiderin-laden foamy macrophages (Fig. 6 and 7). Tariq et al. [8] reported findings consistent with our case where they reviewed H and E-stained microscopic glass slides of 38 GCTB cases who received denosumab as neoadjuvant treatment. In 20 cases, there was a complete absence of osteoclast-type giant cells with stromal changes which included cystic spaces, foamy macrophages, inflammatory infiltrate, hemangiopericytoma- like vessels, hyalinization, edematous areas, and hemosiderin pigment. Yuan et al. showed in their study that after denosumab treatment there was increased internal and peripheral ossification. This was consistent with our case also [9]. This reduced the size of the tumor and made the tumor contained. The thick peripheral wall formed due to denosumab treatment helped in curettage and bone cementing. Weschenfelder et al. showed that the use of denosumab in GCTs with pathological fractures can salvage joints, which is rare. Similarly, in our case, the use of denosumab allowed us to save the proximal femur and joint [10].
In spite of being very rare, GCT is one of the most common primary neoplasms of the bone. It has vcharacteristic histopathological findings which make way for relative ease in said diagnosis. The advent of newer pharmacological agents like denosumab for the treatment of GCT which cause significant improvement in the health condition of the patient also affect the morphology of the tumor. This in turn creates a unique challenge in diagnosing a case post-treatment and makes a thorough workup and clinical history all the more important.
Diagnosing GCTs in achondroplasia patients is challenging due to overlapping histopathology and altered radiological features post-denosumab treatment, requiring careful evaluation for accurate management.
References
- 1.Verschoor AJ, Bovée JV, Mastboom MJ, Sander Dijkstra PD, Van De Sande MA, Gelderblom H. Incidence and demographics of giant cell tumor of bone in The Netherlands: First nationwide Pathology Registry Study. Acta Orthop 2018;89:570-4. [Google Scholar]
- 2.Borkowska AM, Szumera-Ciećkiewicz A, Szostakowski B, Pieńkowski A, Rutkowski PL. Denosumab in giant cell tumor of bone: Multidisciplinary medical management based on pathophysiological mechanisms and real-world evidence. Cancers (Basel) 2022;14:2290. [Google Scholar]
- 3.Van der Heijden L, Dijkstra PD, Blay JY, Gelderblom H. Giant cell tumour of bone in the denosumab era. Eur J Cancer 2017;77:75-83. [Google Scholar]
- 4.Kumar R, Mallya V, Mandal S, Tomar R, Khurana N, Maini L. Histopathological response to denosumab in giant cell tumours of bone - A review of 11 cases. J Cancer Res Ther 2023;19:768-72. [Google Scholar]
- 5.Tariq MU, Umer M, Khan Z, Saeed J, Siddiqui MA, Din NU. Spectrum of histological features of Denosumab treated giant cell tumor of bone; Potential pitfalls and diagnostic challenges for pathologists. Ann Diagn Pathol 2020;45:151479. [Google Scholar]
- 6.McDonald EJ, De Jesus O. Achondroplasia. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/nbk559263 [Google Scholar]
- 7.Gilani A, Kleinschmidt-DeMasters BK. Denosumab therapy obscures histological features of giant cell tumor of bone. J Neuropathol Exp Neurol 2019;78:1171-3. [Google Scholar]
- 8.Li H, Gao J, Gao Y, Lin N, Zheng M, Ye Z. Denosumab in giant cell tumor of bone: Current status and pitfalls. Front Oncol 2020;10:580605. [Google Scholar]
- 9.Yuan B, Han S, Yang S, Zhang L, Jiang L, Wei F, et al. Radiologic and clinical changes after denosumab treatment for giant cell tumors of the mobile spine: A quantitative study. Insights Imaging 2022;13:93. [Google Scholar]
- 10.Weschenfelder W, Abrahams JM, Johnson LJ. The use of denosumab in the setting of acute pathological fracture through giant cell tumour of bone. World J Surg Oncol 2021;19:37. [Google Scholar]