Aneurysmal bone cysts, though seen in younger individuals can manifest in the elderly.
Dr. Mantu Jain, Department of Orthopedics, AIIMS, Bhubaneswar, Odisha, India. E-mail: montu_jn@yahoo.com
Introduction: Aneurysmal bone cyst (ABC) is a rare, benign, cystic, hemorrhagic expansile osteolytic lesion of unknown etiology resembling multiple intraosseous blood-filled spaces mostly seen in the first two decades of life. It accounts for <1% of all bone tumors and the usual sites are the metaphyses of the proximal and distal femur, proximal tibia, and posterior elements of the spine. Affection of other sites is unusual.
Case Report: A 55-year-old female with a 5-month history of pain and swelling of distal ulna. She was operated on with curettage, bone grafting, and plating. At 1-year follow-up, she regained complete movements without any recurrence.
Conclusion: In this article, we present a case of a primary ABC of distal ulna in an unusual age group of 55 years in a female. The clinical presentation, diagnosis, surgical management, and clinical outcome at 1 year are described.
Keywords: Aneurysmal bone cyst, elderly, distal ulna.
Aneurysmal bone cysts (ABC) are benign but locally destructive blood-filled lesions. It results due to the circulatory disturbances within the bone [1]. ABC can be primary (80%) or secondary (20%) to other bone lesions such as giant cell tumors, non-ossifying fibroma, unicameral bone cysts, osteoblastoma, chondroblastoma, chondrosarcoma [1]. Usually occur in the below 20 years age group, with predominance in females [1]. Although it can involve any bone in the body, metaphysis of the distal femur, proximal tibia, proximal humerus, and the posterior elements of the spine are the most frequent locations. The clinical presentation of ABC varies depending on the area of involvement and how aggressive the lesion is. The patient usually presents with mild-to-moderate pain, swelling, deformity, pathological fracture, or any pressure symptoms. Neurological deficits or radiculopathy can be seen in spinal lesions. The radiological appearance of ABC is a lytic, expansile lesion with septations and cortical thinning. fluid–fluid levels seen on magnetic resonance imaging (MRI) [2]. In this article, we present an atypical case of primary ABC of distal ulna in a 55-year-old female. The clinical presentation, surgical management, and clinical outcome at 3 months are described.
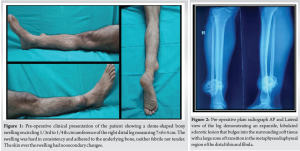
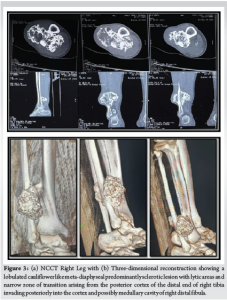
A 55-year-old female presented to our institute with a 5-month history of left wrist pain and swelling. The pain, sudden in onset, was of moderate intensity, exacerbated by movement, and alleviated by rest and medication. There was no history of trauma or fever. Physical examination revealed a non-tender swelling over the distal ulna associated with restricted movement. The overlying skin appeared normal without any signs of injury or vascular compromise, and neurovascular examination was unremarkable. Radiographic imaging demonstrated an expansile lytic lesion with regular margins involving the distal ulnar metaphysis (Fig. 1a). MRI revealed a multi-lobulated space-occupying lesion measuring 29 × 11 × 9 mm, with thinning of the cortex and a pathological fracture along its lateral aspect. The lesion exhibited hypo-intensity on T1-weighted images and hyper-intensity with fluid–fluid levels on T2-weighted images (Fig. 1b-f). Fine needle aspiration and cytology revealed blood. The patient was counseled for surgery.
Through the dorso-medial approach to the distal ulna, curettage of the lesion was done. The bony defect after curettage (Fig. 2a) was filled with bone graft (autograft from the iliac crest) and stabilized with a 1/3rd tubular plate. A curetted sample was sent for histopathological examination, which revealed mature lamellar bone with fibro-collagenous tissue, marrow elements, and areas of hemorrhage. Post-operative radiograph immediately after surgery was shown in Fig. 2b. Range of motion exercises were started at 1 month. At the end of 3 months, the patient regained 70° of flexion and extension. In the final follow-up at 1 year with a loss of terminal wrist joint movements, the patient was pain-free and overall satisfied with the result (Fig. 3a and b). X-ray shows some resorption of the graft and collapse (Fig. 3c). The patient refused for any further procedure (implant removal).
ABC is a rare, benign, cystic, hemorrhagic, expansile, and osteolytic lesion of unknown etiology characterized by multiple intraosseous blood-filled spaces, typically observed in individuals within the first two decades of life. Eighty percent of ABCs occur in patients younger than 20 years [1]. The occurrence of ABC in patients over 50 years old is exceedingly uncommon, as highlighted in our unique case report. Representing only 1–2% of all bone tumors, ABCs predominantly manifest in the metaphysis of long bones such as the tibia, femur, and humerus, with approximately 10% occurring in the pelvis [2,3]. Notably, involvement of the ulna is particularly rare, with only a limited number of documented cases in the existing literature. Our case report presents a distinctive instance of ABC affecting the distal ulna, further underscoring the rarity of this presentation. ABCs can be classified as either primary (80%) or secondary (20%) to other bone lesions, including giant cell tumors, non-ossifying fibroma, unicameral bone cysts, osteoblastoma, chondroblastoma, and chondrosarcoma [3]. Among these secondary cases, giant cell tumors are the most common, accounting for 19–39% of cases [1]. The pathogenesis of ABCs is still not fully understood. However, several theories have been proposed, including trauma, vascular malformations, and reactive bone changes. In a recent study, ubiquitin-specific protease 6 (USP6) gene rearrangement was found to be associated with 65–70% of cases, whereas CDH11-USP6 fusion was associated with 30% of cases [4]. The translocated USP6 oncogene blocks the osteoblastic maturation, causing ABC [5]. It also increases the matrix metalloproteinases (MMPs), causing degradation of the extracellular matrix, which results in the expansion of ABC lesions [6]. The clinical presentation of ABCs is variable. Patients may present with pain, swelling, tenderness, or a palpable mass. The symptoms are often related to the size and location of the lesion. In some cases, ABCs may be asymptomatic and discovered incidentally on imaging studies performed for other reasons. The characteristic macroscopic features of ABCs are their blood-filled cystic spaces, surrounded by a thin layer of reactive bone. These cystic cavities are often described as “blood-filled lakes” separated by delicate, fibrous septae. While predominantly cystic, solid areas within the lesion are also observed. Microscopically, ABCs demonstrate a heterogeneous structure. The most prominent features are the large blood-filled cystic spaces, lacking any epithelial or endothelial lining [4]. Radiographically, ABCs typically present as eccentric, metaphyseal osteolytic lesions with minimal marginal sclerosis or septation. The classic radiographic hallmark of ABCs is the “blowout” phenomenon, characterized by an expansile, ballooned-out lesion outlined by a thin shell of periosteal new bone formation. This “blowout” appearance is a result of the pressure exerted by the blood-filled cystic spaces within the lesion, leading to the expansion and thinning of the cortical bone [7]. MRI findings consist of a hypointense rim surrounding the lesion with multiple intralesional septations and cysts with fluid–fluid levels of different intensities [8]. However, the diagnosis is always confirmed by a biopsy. There are various treatment options for ABC reported in the literature; however, curettage with or without bone grafting is the mainstay of treatment. Despite improved curettage techniques, recurrence rates are still significant, reaching as high as 59% [9]. Various adjunctive therapies have been explored to mitigate recurrence, including high-speed burring, phenol injection, bone cement, argon beam laser therapy, cryotherapy, and liquid nitrogen. However, definitive evidence is lacking supporting the superiority of one adjunctive technique over another. Some studies have demonstrated promising results with the use of a high-speed burr. Gibbs et al. and Dorman et al. reported reduced recurrence rates of 10% and 18%, respectively, with this approach [10-12]. In the present case, a high-speed burr was employed during curettage to effectively break down the septations within the lesion. This approach was chosen based on its potential benefits in completely removing the cystic contents and reducing the likelihood of recurrence. Kececi et al. reported no significant difference in recurrence rates after using phenol when compared to curettage with high-speed burr only [13]. Due to its high complication rates such as skin necrosis, post-operative fractures, and wound infection, cryosurgery is not a preferred adjuvant for the treatment of ABC [11]. A bone graft is mostly used to fill the defect following curettage. Bone cement can also be used to fill the defect, reducing recurrence rates by killing the cancer cells through its exothermic effect. A study by Ozaki and colleagues found that the recurrence rate was lower when curettage and cementing were utilized compared to curettage and grafting alone, with rates of 17% and 37%, respectively [14]. Another retrospective comparative study focusing on the impact of cement versus bone grafting in benign pediatric bone lesions, conducted by Wallace et al. revealed comparable complication and recurrence rates. Similarly, Mankin et al. also reported similar recurrence rates, whether bone grafting or cement was employed [3,15]. Due to the lack of supportive evidence, we preferred to use a bone graft over bone cement to fill the bone defect in our case. Apart from surgical treatment, various non-surgical techniques have recently gained popularity, such as sclerotherapy, percutaneous doxycycline injection, and arterial embolization. The use of various sclerosing agents such as polidocanol, ethanol, and doxycycline has been reported in the literature. Kumar et al., in their case series of 26 patients, reported recurrence in two patients and also reported local complications such as skin induration and hypopigmentation in a few patients [16]. Cruz and colleagues reported that 68 out of the 294 patients experienced complications, including issues such as injection site induration, skin necrosis, and fractures [17]. Although sclerotherapy has shown promising results, long-term studies are still required for widespread use. The efficacy of denosumab as a neoadjuvant therapy for giant cell tumors is well-documented, but its utility in ABC remains less defined. Palmerini et al. conducted an observational study that yielded positive outcomes with the use of Denosumab in ABC patients, recommending its application specifically in cases of uncontrollable, locally destructive, and recurrent ABCs [18].
This case highlights the rarity of an ABC occurring in the distal ulna of an adult patient and underscores the successful outcome achieved through extended curettage and bone grafting. These findings contribute to the limited literature on the management of ABC in this unique anatomical location and provide valuable insights for clinicians considering treatment options for similar cases.
ABC can manifest in the elderly. Therefore, extensive workup and proper diagnosis are crucial to its management.
References
- 1.Kransdorf MJ, Sweet DE. Aneurysmal bone cyst: Concept, controversy, clinical presentation, and imaging. AJR Am J Roentgenol 1995;164:573-80. [Google Scholar]
- 2.Shukla R, Jain N. Aneurysmal bone cyst-an unusual presentation of wrist pain. J Wrist Surg 2019;8:76. [Google Scholar]
- 3.Mankin HJ, Hornicek FJ, Ortiz-Cruz E, Villafuerte J, Gebhardt MC. Aneurysmal bone cyst: A review of 150 patients. J Clin Oncol 2005;23:6756-62. [Google Scholar]
- 4.Restrepo R, Zahrah D, Pelaez L, Temple HT, Murakami JW. Update on aneurysmal bone cyst: Pathophysiology, histology, imaging and treatment. Pediatr Radiol 2022;52:1601-14. [Google Scholar]
- 5.Lau AW, Pringle LM, Quick L, Riquelme DN, Ye Y, Oliveira AM, et al. TRE17/ubiquitin-specific protease 6 (USP6) oncogene translocated in aneurysmal bone cyst blocks osteoblastic maturation via an autocrine mechanism involving bone morphogenetic protein dysregulation. J Biol Chem 2010;285:37111-20. [Google Scholar]
- 6.Ye Y, Pringle LM, Lau AW, Riquelme DN, Wang H, Jiang T, et al. TRE17/USP6 oncogene translocated in aneurysmal bone cyst induces matrix metalloproteinase production via activation of NF-kappaB. Oncogene 2010;29:3619-29. [Google Scholar]
- 7.Ulici A, Nahoi C, Carp M, Fodor I, Dinu C. Surgical treatment of an aneurysmal bone cyst with avascular bone graft. Chirurgia (Bucur) 2017;112:172-7. [Google Scholar]
- 8.Beltran J, Simon DC, Levy M, Herman L, Weis L, Mueller CF. Aneurysmal bone cysts: MR imaging at 1.5 T. Radiology 1986;158:689-90. [Google Scholar]
- 9.Biesecker JL, Marcove RC, Huvos AG, Miké V. Aneurysmal bone cysts. A clinicopathologic study of 66 cases. Cancer 1970;26:615-25. [Google Scholar]
- 10.Gibbs CP, Hefele MC, Peabody TD, Montag AG, Aithal V, Simon MA. Aneurysmal bone cyst of the extremities. Factors related to local recurrence after curettage with a high-speed burr. J Bone Joint Surg Am 1999;81:1671-8. [Google Scholar]
- 11.Park HY, Yang SK, Sheppard WL, Hegde V, Zoller SD, Nelson SD, et al. Current management of aneurysmal bone cysts. Curr Rev Musculoskelet Med 2016;9:435-44. [Google Scholar]
- 12.Lin PP, Brown C, Raymond AK, Deavers MT, Yasko AW. Aneurysmal bone cysts recur at juxtaphyseal locations in skeletally immature patients. Clin Orthop 2008;466:722-8. [Google Scholar]
- 13.Keçeci B, Küçük L, Isayev A, Sabah D. Effect of adjuvant therapies on recurrence in aneurysmal bone cysts. Acta Orthop Traumatol Turc 2014;48:500-6. [Google Scholar]
- 14.Ozaki T, Hillmann A, Lindner N, Winkelmann W. Cementation of primary aneurysmal bone cysts. Clin Orthop 1997;337:240-8. [Google Scholar]
- 15.Wallace MT, Henshaw RM. Results of cement versus bone graft reconstruction after intralesional curettage of bone tumors in the skeletally immature patient. J Pediatr Orthop 2014;34:92-100. [Google Scholar]
- 16.Kumar D, Kumar S, Kumar D, Patel BM, Kumar A, Kumar S, et al. Sclerotherapy for aneurysmal bone cyst: A single-center experience. Cureus 2021;13:e18469. [Google Scholar]
- 17.Cruz GS, Cuevas-Suárez CE, Saavedra JP, Giorgis R, Teixeira MR, Muniz FW. Percutaneous treatments of primary aneurysmal bone cysts: Systematic review and meta-analysis. Eur J Orthop Surg Traumatol Orthop Traumatol 2021;31:1287-95. [Google Scholar]
- 18.Palmerini E, Ruggieri P, Angelini A, Boriani S, Campanacci D, Milano GM, et al. Denosumab in patients with aneurysmal bone cysts: A case series with preliminary results. Tumori 2018;104:344-51. [Google Scholar]