Heightened vigilance and prompt surgical intervention are crucial in managing acute compartment syndrome (ACS) caused by iatrogenic fluid extravasation, especially in patients unable to communicate their symptoms.
Dr. Joseph Vyskocil, Central Michigan University School of Medicine, 1632 Stone St, Saginaw, MI 48602. E-mail: vysko1j@cmich.edu
Introduction: Acute compartment syndrome (ACS) in the hand and forearm is an uncommon yet significant orthopedic crisis. The misplacement or migration of an intravenous (IV) catheter can cause fluid extravasation into interstitial tissues, which is a rare but known cause of ACS. Diagnosis of ACS is usually clinical, but this can be challenging in anesthetized or obtunded patients who are unable to communicate. Management involves emergent fasciotomies to relieve compartment pressures.
Case Report: We present the case of a 64-year-old female who developed ACS in the hand and forearm following a carotid endarterectomy. The patient experienced significant swelling in her left hand and forearm due to an infiltrated IV catheter. The diagnosis was based solely on physical examination, as the patient was under anesthesia. Findings included swelling, tissue tension, and color changes suggestive of early ischemia. Emergent fasciotomies were performed to decompress the affected compartments.
Conclusion: ACS resulting from iatrogenic fluid extravasation is a severe condition with a rare etiology. Prompt identification and surgical intervention are essential to reduce associated morbidity and mortality. In certain cases, the diagnosis must be made by physical examination alone. The rarity of this etiology necessitates heightened awareness among inpatients who have a limited ability to communicate.
Keywords: Acute compartment syndrome, iatrogenic fluid extravasation, emergent fasciotomies.
Acute compartment syndrome (ACS) is a critical condition necessitating emergent surgical decompression to prevent permanent soft tissue injury, neurologic dysfunction, and disability. Typically, ACS is identified and diagnosed based on clinical evaluation.[1] However, a difference (DP) between intracompartmental pressure (ICP) and diastolic blood pressure (DP) of <30 mmHg can serve as a diagnostic threshold to assist in identifying the condition.[2] It is important to note, however, that a single normal DP measurement does not rule out the presence of ACS. Although trauma is the most frequent cause, other less prevalent etiologies such as burns, thromboembolism, and iatrogenic injuries also present significant challenges in diagnosis and can be equally problematic.[3] Several studies have indicated a connection between intravenous (IV) extravasation and the development of compartment syndrome, yet the available data on this topic remains sparse. In cases of IV infiltration, misplacement or migration of the catheter results in fluid extravasation into the interstitial soft tissues. This accumulation of fluid in the interstitial space can precipitate the development of compartment syndrome. A recent systematic review identified 51 cases of compartment syndrome related to IV infiltration, with 20 of these involving the hand.[4] The predominant risk factor for this type of compartment syndrome was age, as 40% of the cases were found in pediatric patients.[4,5] Other risk factors included individuals with impaired communication capabilities, such as those with altered mental status or on mechanical ventilation.[6] This case report details the onset and surgical management of intraoperative compartment syndrome in the hand and forearm following extravasation of fluid secondary to an infiltrated IV catheter.
A 64-year-old female with a history of left carotid artery stenosis was taken for an outpatient left carotid endarterectomy. Immediately following the procedure, the anesthesiologist discovered that the left upper extremity IV line had infiltrated and resulted in significant swelling of the left hand and forearm. Orthopedic surgery was consulted emergently and evaluated the patient while the patient remained intubated. The hand was posturing in the intrinsic minus position. The entire volar, dorsal, thenar, and hypothenar aspects of the hand were swollen and tense to palpation. The fingertips appeared dusky and ischemic, and loss of palmar concavity was appreciated. Passive range of motion of the fingers was limited secondary to swelling and the entire hand was pale. Volar, dorsal, and mobile wad compartments of the forearm were firm and tense (Fig. 1). The upper arm compartments remained soft and compressible, and the extravasated fluid did not appear to cross the elbow flexion crease. ACS was diagnosed based on physical examination alone, and the patient’s husband consented emergently for left upper extremity fasciotomies.
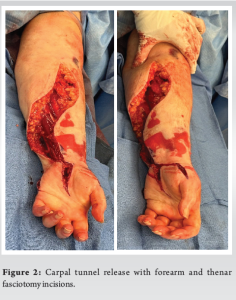
The surgical intervention began with an open carpal tunnel release. A longitudinal incision was made beginning in the mid palm and extending down to the distal aspect of the wrist flexion crease just ulnar to the palmaris longus. Significant amounts of interstitial fluid were encountered. Dissection was carried down to the transverse carpal ligament which was released, and the median nerve was visualized. The median nerve appeared healthy and not erythematous. Proximally the antebrachial fascia was released as well. Following this initial release, the fingers rapidly re-perfused with a capillary refill time of 3 seconds. The carpal tunnel release was then extended distally in a radial curvilinear fashion to decompress the ulnar aspect of the thenar compartment. The incision was also extended ulnarly across the wrist flexion crease, transitioning into a lazy S incision traversing the forearm to cross the flexor and mobile wad compartments, and terminating just distal to the radial aspect of the elbow flexion crease (Fig. 2). Substantial interstitial fluid was again encountered. Blunt dissection was carried down to the flexor compartment fascia, which was tense and rigid to palpation. A sharp incision of the fascia from the wrist to the elbow flexion crease was performed using Metzenbaum scissors. Following the volar fasciotomy, the fingers maintained a pink hue, and the capillary refill improved further to 2 seconds. The volar compartment was confirmed to be soft and adequately decompressed.
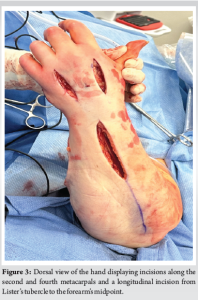
Satisfied with the results of the volar forearm fasciotomy, focus was redirected once again to the hand. Longitudinal incisions were made over the radial aspect of the thenar compartment and over the ulnar aspect of the hypothenar compartment (Fig. 2). The thenar and hypothenar compartments were both decompressed and remained soft thereafter. Further, two dorsal incisions were made in line with the second and fourth metacarpals to decompress the adductor and interosseous compartments, leading to the expression of a cloudy fluid, likely infiltrated propofol, from the dorsum of the hand (Fig. 3). The extensor tendons were examined and found to be intact. A final longitudinal dorsal incision along the forearm was made, extending from just proximal to Listers tubercle to the midpoint of the forearm (Fig. 3). This allowed for visualization and decompression of the forearm extensor compartment. Upon reevaluation, all compartments were found to be soft and compressible, and the hand demonstrated good perfusion and warmth, with a strong dopplerable ulnar pulse. The radial pulse was not Dopplerable at this stage. The capillary refill time at this stage was 1.5 s. All wounds in the hand and the dorsal aspect of the forearm were closed with nylon sutures, whereas the volar forearm fasciotomy was left open and covered with the application of negative pressure wound therapy. The patient underwent subsequent partial closure of her volar forearm wound on post-operative day 4, and plastic surgery performed split-thickness skin grafting of the remaining open wound on post-operative day 7.
Compartment syndrome is predominantly diagnosed by physical examination, classically described as the five P’s – pain, pallor, paresthesia, paralysis, and pulselessness. Given the variability in patient presentation, the most dependable signs are pain during passive stretch and pain out of proportion to the injury, as signs of neurovascular compromise are typically later in the disease course.[5] However, in the intubated, obtunded, or mentally ill patient, the ability of one to communicate the onset and degree of pain may be compromised, leading clinicians to resort to alternative methods of diagnosing ACS. The occurrence of compartment syndrome under anesthesia is uncommon, yet it can lead to severe and significant complications, primarily due to delays in diagnosis.[7] This case highlights the importance of a diagnosis and treatment based on physical examination in the communication-impaired or anesthetized patient. Patients receiving general anesthesia or epidurals for lower extremity surgery must be closely observed for the development of ACS. Iaquinto and Strecker independently quoted a 4-times higher risk of missed compartment syndrome in those undergoing tibial fracture fixation who received a post-operative epidural compared to those who received narcotics only.[8] In addition to analgesia, epidurals are known to increase local blood flow secondary to sympathetic blockade and can lead to increased swelling of an injured limb. Harrington described a case of patient-controlled analgesia (PCA) that masked symptoms of a developing compartment syndrome.[9] While patients sustaining traumatic injuries, especially those at higher risk of developing ACS (e.g., crush injuries) present with a higher pre-operative probability of developing ACS, this may not always be the case in the non-traumatic patient. Garner et al. attributed fracture as the most common cause of ACS (69%), with 30% specifically associated with tibial shaft fractures.[3] Male gender and age < 35 years were associated risk factors for ACS; however, this was only true in traumatic cases. Iatrogenic causes, including IV infiltration, are much less common and extravasation of fluids or drugs leading to ACS is a rare complication infrequently documented in the available literature.[4] Most cases of IV fluid leakage into surrounding tissues are minor and respond well to non-invasive treatments such as elevation of the affected limb and allowing time for fluid reabsorption.[10] However, close observation and judicious use of compartment pressure measuring devices are paramount in diagnosing ACS in communication-impaired patients, with a low threshold for surgical intervention, especially in diagnostically ambiguous cases. Pare and Moore systematically reviewed 51 cases of ACS secondary to IV infiltration. Of the 42 cases where mental status was reported, 76.2% were deemed to have altered mentation, impaired sensation, or were younger than 3 years.[4] The definitive approach to managing ACS involves performing emergent fasciotomies, with incisions strategically made to decompress all affected compartments.[11] Although fasciotomies are associated with low morbidity, the sequelae of missed compartment syndrome are substantial, including lifelong disability or death.[12] While the incidence of ACS secondary to IV fluid extravasation remains a far less common etiology, in the setting of non-classic risk factors the surgeon’s clinical suspicion must remain high, especially in the communication-impaired patient. We believe this case adds to the existing literature supporting the clinical vigilance and emergent surgical treatment necessary in iatrogenic cases of ACS secondary to IV fluid extravasation.
ACS secondary to iatrogenic fluid extravasation is a rare but devastating condition necessitating prompt examination and diagnosis followed by emergent surgical decompression. The rarity of this specific etiology must be well-appreciated by health-care professionals across various specialties, especially those caring for patients whose ability to communicate pain may be impaired. Medical specialties including anesthesia, intensive care, emergency medicine, pediatrics, and geriatrics may be most likely to care for patients with impaired communication or sensation and should consider this complication when treating patients with IV complications. Education and communication among care providers are essential for the early detection and timely treatment of compartment syndrome.
Prompt recognition and surgical intervention are essential for managing ACS caused by iatrogenic fluid extravasation, particularly in patients who cannot communicate their symptoms. Early detection through thorough physical examination and effective collaboration among health-care providers is critical to prevent severe outcomes.
References
- 1.Walters TJ, Kottke MA, Hargens AR, Ryan KL. Noninvasive diagnostics for extremity compartment syndrome following traumatic injury: A state-of-the-art review. J Trauma Acute Care Surg 2019;87:S59-66. [Google Scholar]
- 2.Torlincasi AM, Lopez RA, Waseem M. Acute compartment syndrome. In: StatPearls. StatPearls Publishing; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK448124 [Last accessed on 2023 Dec 08]. [Google Scholar]
- 3.Garner MR, Taylor SA, Gausden E, Lyden JP. Compartment syndrome: Diagnosis, management, and unique concerns in the twenty-first century. HSS J 2014;10:143-52. [Google Scholar]
- 4.Pare JR, Moore CL. Intravenous infiltration resulting in compartment syndrome: A systematic review. J Patient Saf 2018;14:e6-8. [Google Scholar]
- 5.Barber CM, Fahrenkopf MP, Adams NS, Kelpin JP, Krebiehl JR. Compartment syndrome of the hand secondary to intravenous extravasation. Eplasty 2018;18:ic17. [Google Scholar]
- 6.Ouellette EA. Compartment syndromes in obtunded patients. Hand Clin 1998;14:431-50. [Google Scholar]
- 7.Araz C, Cetin S, Didik M, Balli Seyhan S, Komurcu O, Arslan G. A case of compartment syndrome in the hand secondary to intravenous fluid application. Turk J Anesth Reanim 2015;43:126-9. [Google Scholar]
- 8.Taylor RM, Sullivan MP, Mehta S. Acute compartment syndrome: obtaining diagnosis, providing treatment, and minimizing medicolegal risk. Curr Rev Musculoskelet Med 2012;5:206-13. [Google Scholar]
- 9.Harrington P, Bunola J, Jennings AJ, Bush DJ, Smith RM. Acute compartment syndrome masked by intravenous morphine from a patient-controlled analgesia pump. Injury 2000;31:387-9. [Google Scholar]
- 10.Stavrakakis IM, Daskalakis II, Detsis EP, Karagianni CA, Papantonaki SN, Katsafarou MS. Hand compartment syndrome as a result of intravenous contrast extravasation. Oxf Med Case Rep 2018;2018:omy098. [Google Scholar]
- 11.Oak NR, Abrams RA. Compartment syndrome of the hand. Orthop Clin North Am 2016;47:609-16. [Google Scholar]
- 12.Lim KB, Laine T, Chooi JY, Lye WK, Lee BJ, Narayanan UG. Early morbidity associated with fasciotomies for acute compartment syndrome in children. J Child Orthop 2018;12:480-7. [Google Scholar]