Two-stage revision arthroplasty is still the gold standard for managing late periprosthetic joint infections, even in socioeconomically disadvantaged patients.
Prof. Dr. Pervez Ahsan, Department of Orthopedic Surgery, Ibn Sina Medical College and Hospital, Dhaka, Bangladesh. E-mail: pervezahsan60@yahoo.com
Introduction: Periprosthetic joint infections (PJIs) present a growing challenging complication in joint surgeries, with increasing incidence and significant clinical implications. The complexity of PJIs lies in its interaction between microbes and the host immune response. While Enterobacter infections are rare in PJIs, they pose severe risks due to their resistance and virulence. PJIs burden healthcare systems and profoundly impact patients, especially those unable to access necessary treatments due to socioeconomic limitations, like our patient who had suffered from excruciating pain and immobility for 6 years. To the best of our knowledge, this is the first case report of its kind.
Case Report: A 55-year-old woman presented to us with severe right hip pain for 10 years and recurrent swelling over the incisional site with discharging sinus and intermittent fever for 6 years. She underwent through a unipolar hemiarthroplasty due to right-sided femoral neck fracture 10 years back. Severe pain and recurrent infections led to functional limitations and deteriorating quality of life. History, clinical examination, serological, and radiological investigations confirmed the diagnosis. Two-stage revision total hip arthroplasty was performed. Regular follow-ups were done after the surgical procedure. After revision, she experienced no pain or recurrence of infection. Her Harris Hip Scores were 9 and 80 before and after two-stage revision surgery, indicating a favorable outcome.
Conclusion: This case highlights the challenges of PJI management, especially in patients from poor socioeconomic condition. Despite obstacles, intervention with two-stage revision arthroplasty resulted in significant clinical and psychological improvement. Enhanced healthcare accessibility and tailored interventions are crucial for optimizing PJI outcomes.
Keywords: Periprosthetic joint infection, revision total hip replacement, unipolar hemiarthroplasty, osteoporosis, Harris Hip Score.
Periprosthetic joint infection (PJI), also known as arthroplasty-associated infection, poses a significant challenge following total joint arthroplasty (TJA) [1]. PJI is characterized by an infection affecting both the joint prosthesis and the surrounding tissue [2]. Its occurrence is noted in 1–2% of primary and in 4% of revision arthroplasties [3]. With the escalating number of TJA procedures performed among the elderly population, so it is expected that the incidence of PJI will grow in the coming years [4, 5]. The incidence of PJI ranges from 0.25% to 2.0% [6]. Specifically, PJI frequencies are estimated at 1% for total hip replacement (THR) and 2% for total knee replacement (TKR) surgeries [7]. However, in cases necessitating revision surgery, the infection rates increased to 3% for THR and 5% for TKR [7]. PJIs are a unique clinical entity, markedly differing from infections affecting native bones or joints [8]. It involves a complex interaction between microbes, predominantly bacteria but occasionally fungi, and the host immune response [8]. Even a small number of microbes is sufficient to trigger a PJI, as these organisms can adhere to the surfaces of artificial joint components and form biofilms [8]. Biofilms, which are complex structures consisting of microorganisms surrounded by glycocalyx and other protective substances, play a crucial role in the development of PJI [9, 10]. Bacterial attachment to surfaces occurs through cell-to-cell adhesion between microorganisms and the artificial surface [10]. The majority of PJIs are caused by Gram-positive cocci, notably Staphylococcus aureus and coagulase-negative Staphylococcus [10]. A history of prior surgery at the arthroplasty site, current bacteremia or sepsis, and a previous or active infection at the current surgical site, pre-operative anemia, poor pre-operative glycemic control, first- or second-degree relative with a history of PJI, smoking, alcohol abuse, intravenous drug use, poor oral hygiene, malnutrition, morbid obesity, uncontrolled diabetes mellitus, cardiovascular disorders, acute liver injury, chronic renal failure, inflammatory arthropathies, depression, infection with the human immunodeficiency virus and the use of immunosuppressant (corticosteroids) or disease-modifying antirheumatic agents elevate the risk of developing PJIs [8, 11]. Moreover, limited adoption of evidence-based PJI preventive measures significantly increases the susceptibility of PJI [8]. Surgical factors also contribute to the risk profile, with prolonged operative durations exceeding 90 min and increasing procedural complexity amplifying the likelihood of PJI occurrence [8]. Notably, patients with hip dysplasia undergoing arthroplasty face a markedly elevated risk of PJI [12]. Furthermore, individuals undergoing primary arthroplasty who experienced post-operative complications such as hematoma, seroma, or wound dehiscence are at heightened risk of developing PJI [8]. Early PJIs occur within the initial 4 weeks, delayed PJIs occur between 3 and 12 months, and late PJIs occur 1–2 years following the primary arthroplasty [8]. In a Canadian population-based study, the incidence of hip PJI was 0.5% at 1 year and 1.4% at 15 years [13]. The most common symptom is pain in the affected joint [14]. Local inflammatory signs include erythema, swelling, and warmth of the joint [14]. Systemic manifestations of infection, such as fever, are frequently absent [8]. Chronic infections may present subtly, with pain as the sole symptom, or conspicuously when associated with prosthetic loosening or a draining sinus tract [8]. While a draining sinus is pathognomonic for PJI, its absence does not preclude the diagnosis [8]. To diagnosis PJI, a clear definition is required. The 2018 Musculoskeletal Infection Society definition of hip and knee PJI is evidence-based and validated criteria [15]. It encompasses major and minor criteria [15]. Major criteria include the presence of at least one major criterion, such as two positive cultures of the same organism or the presence of a sinus tract communicating with the joint, both of which indicate infection [15]. Minor criteria, with their respective scores, consist of elevated C-reactive protein (CRP) or D-dimer (score: 2), elevated erythrocyte sedimentation rate (ESR) (score: 1), elevated synovial white blood cell (WBC) count or leukocyte esterase (score: 3), positive alpha-defensin (score: 3), elevated synovial PMN (%) (score: 2), elevated synovial CRP (score: 1) [15]. Pre-operative scores of minor criteria 6 or higher suggest infection, 4-5 are inconclusive (possibly infected), and 3 or less indicate no infection [15]. For patients with inconclusive minor criteria, operative criteria can also be used to fulfill the definition for PJI [15]. Intraoperative diagnosis relies on positive histology (score: 3), positive purulence (score: 3), and a single positive culture (score: 2), with a decision threshold indicating infection if the pre-operative score is 6 or higher, inconclusive if it is 4-5, and no infection if it is 3 or less [15]. Further molecular diagnostics such as next-generation sequencing may be considered for inconclusive cases [15]. Imaging studies include plain radiograph, computed tomography, and magnetic resonance imaging [3]. Debridement, antibiotics, and implant retention may be utilized to treat acute PJI, unless there is a sinus tract, the prosthesis is loose, or the wound cannot be closed [8]. Chronic infections necessitate resection arthroplasty, either one or two-stage revisions [14]. For decades, two-stage revision surgery has been considered the “gold standard” [16]. PJI is associated with a high financial burden on the healthcare system, as well as significant physical and psychological morbidity in patients [2]. We discussed the journey of a 55-year-old woman grappling with late periprosthetic infection (PJI) of the right hip, stemming from a femoral neck fracture managed with unipolar hemiarthroplasty. Through a two-stage revision total hip arthroplasty (reTHA) and a tailored antibiotic regimen, she experienced a remarkable recovery, transitioning from bedridden to independent ambulation. This report not only sheds light on the challenges faced by socioeconomically disadvantaged patients in accessing timely surgical care but also underscores the feasibility and efficacy of reTHA in improving outcomes for late PJIs.
A 55-year-old woman presented to us with long-standing, severe right hip pain persisting for 10 years, accompanied by recurrent swelling over the incisional site with discharging sinus and intermittent fever for the past 6 years. 10 years back, she underwent unipolar hemiarthroplasty of the right hip following a femoral neck fracture sustained from a fall. This surgery was performed in a local low-profile clinic in Dhaka. Unfortunately, her post-operative period was complicated by persistent pain unresponsive to medication and physiotherapy. Consequently, she experienced significant functional limitations, requiring the use of a cane for ambulation and confining her mobility to her residence. These challenges had a profound impact on her daily activities, leading to feelings of depression. 6 years ago, she noticed an indurated, black-colored swelling developed at the surgical incision site, accompanied by purulent discharge and intermittent febrile episodes with a maximum body temperature of 101° F. She visited the local clinic and received multiple courses of antibiotics, but the swelling with discharging sinus recurred frequently. She became bedridden, and her quality of life deteriorated day by day. After consulting many orthopedic surgeons, she was diagnosed with PJI. Pus culture and sensitivity identified Enterobacter as the causative organism. They recommended revision THR, but as a widow with no children, unemployed, and very poor economic condition, she could not afford the procedure. Over the past 3 years, her pain became excruciating. She came to our outpatient department for further consultation. On arrival, the patient was found to be completely bedridden, with a lean and thin body build. Her body temperature was recorded at 100°F. Examination of the right hip revealed severe tenderness, swelling, and a sinus tract over the incisional site, with a slight oozing of yellowish pus. The Harris Hip Score (HHS) on presentation was 9, indicative of poor hip status. She was normotensive and non-diabetic.
Laboratory markers
Laboratory investigations revealed a hemoglobin level of 9.5 g/dL, ESR of 57 mm, a WBC count of 6,680/mm3, with neutrophils comprising 51% and lymphocytes 43%, and a CRP level of 11.3 mm/L. Pus for culture and sensitivity conducted twice identified Enterobacter species that were resistant to amoxiclav, cefixime, ceftriaxone, and cefuroxime. All other pre-operative blood tests were within normal ranges.
Radiological findings
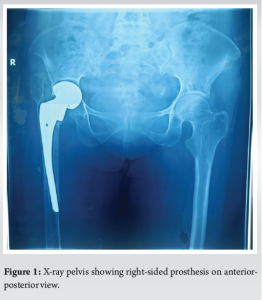
X-ray right pelvis showing no lesser trochanter, greater trochanter tilted upward and laterally, absence of lateral cortex of femur, prosthetic tip was migrated medially, and the acetabular surface was eroded (Fig. 1). In addition, bone mineral density (BMD) measurements revealed spine BMD of −3.9 and femur BMD of −4.3, indicating osteoporosis.
Diagnosis and treatment plan
After proper medical history, clinical examination, serological, and radiological investigations, she was diagnosed as a late case of periprosthetic infection of the right hip. We planned to do two-stage reTHA.
Surgical procedure
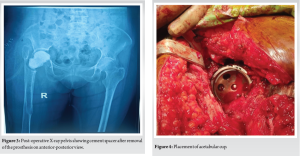
Stage one: After maintaining all aseptic precautions, we employed a lateral approach. The greater trochanter was identified with prosthesis. Greater trochanter was severely osteoporotic. There was a chance of fracture while removal of the implant. Dislocation of head of prosthesis and removal of it were done successfully (Fig. 2). Acetabular surface was deficit near 30%. Removal of surrounding tissues was done carefully. After that, we kept an antibiotic-mixed cement as a spacer (Fig. 3) and gave vancomycin powder within the acetabular cavity. After closure, we planned to perform the 2nd stage of surgery 4 weeks later. Stage two: Due to the unavailability of the implant, we had done 2nd stage of surgery 10 weeks (2.5 months) later. We used the same lateral approach with extension up to mid-thigh and removed the cement spacer. We reamed the acetabulum very carefully and found the bone very soft and fragile. For this reason, a non-cemented cup was inserted (Fig. 4) and fixed with three screws. Shaft of the femur was exposed properly and found absence/deficient of the lateral cortex of it. Prosthetic end of medullary cavity was found blocked by bone. Initially, we tried to reaming the medullary cavity under fluoroscopy guided by 7 mm reamer. It was very difficult to achieve the reaming portion at the blocked area. Introduction of stem was difficult due to the chance of re-fracture and there was a big gap, measuring 8 cm × 1.25 cm at the lateral cortex of the femur. To cover this gap, the mid-third of the fibula (harvested by fibulectomy) (Fig. 5) was used as a bone graft, which was then grafted and fixed using surgical wiring (Fig. 6). Afterward, we faced difficulty during cementing technique. During introduction of cement into the femoral medullary cavity, cement was found leaked from the space of the grafted bone. Then leakage was carefully protected. Femoral stem was introduced carefully so that it was be in a position to medullary cavity (Fig. 7). A drain tube was kept in situ, then with aseptic precautions, we closed the surface.


Post-operative results
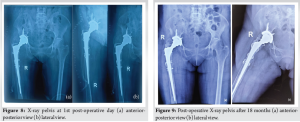
Following the first stage of surgery, the patient was given a combination of piperacillin and tazobactam intravenously, initially at a dose of 4.5 g/vial and then 2.25 g/vial 3 times daily for 14 days. In addition, ciprofloxacin was given orally at a dose of 500 mg twice daily for 1.5 months. She was released on the 7th post-operative day. Following the second stage of surgery, the patient was given intravenous piperacillin and tazobactam, initially at a dose of 4.5 g/vial and then 2.25 g/vial 3 times daily for 7 days. Amikacin was also given intravenously at a dose of 500 mg/mL 3 times daily for 3 days, followed by oral ciprofloxacin at a dose of 500 mg twice daily for 1.5 months. Drainage through the drain tube continued up to the 5th post-operative day. The patient remained hospitalized for 30 days following reTHA to ensure proper physiotherapy, prevent refracture due to osteoporosis, and reduce the risk of reinfection. During the recovery period, the patient received oral analgesic treatment and physiotherapy, leading to a progressive improvement in mobility. Initially relying on a walker for non-weight-bearing ambulation, the patient progressed to using canes and eventually achieved independent walking without assistance. At the 18-month follow-up, the HHS was 80, suggesting good hip status and significant improvement. She gradually resumed her normal daily activities. Post-operative X-rays of the pelvis including both A/P view and lateral view revealed that the mechanical axis was maintained and there was no loosening of the implant (Fig. 8 & 9).
Several studies discuss PJIs. Vergison et al. detailed the case of a 73-year-old woman who developed PJI 1 month after THR in her right hip [17]. Mencia et al. featured an 83-year-old woman who developed PJI a year after a cemented bipolar hemiarthroplasty in her right hip [18]. Athanasiou et al. presented a case involving a 82-year-old woman who developed PJI 9 years after a THR in her right hip [19]. Kawakami et al. described a 79-year-old woman who developed PJI in right hip 28 years after bilateral THR [20]. Ramanathan and Ayoade reported on a 58-year-old man who developed PJI 1 month after a THR in his right hip [21]. In our study, we present a case of a 55-year-old woman who developed PJI 4 years after an unipolar hemiarthroplasty in her right hip. Various studies identified different causative organisms. Vergison et al. identified Candida parapsilosis [17], Athanasiou et al. found Listeria monocytogenes [19], Kawakami et al. detected Sneathia sanguinegens [20], Ramanathan and Ayoade found Mycobacterium fortuitum [21], in our study, we found Enterobacter species, and although multiple samples were taken, Mencia et al. did not find any microorganism [18]. Enterobacter species is a Gram-negative bacillus frequently exhibiting drug resistance, typically constitutes a less common pathogen in PJI [22]. As per Prado et al., the outcomes observed in their cohort underscore the virulence of Enterobacter, marked by elevated failure rates and the necessity for joint- or limb-sacrificing procedures [22]. Although Enterobacter infections in PJIs are infrequent, the prognosis remains notably poor with conventional treatment approaches, in contrast to historical cohorts dominated by more common pathogens [22]. Vergison et al. [17], Athanasiou et al. [19], and we performed two-stage revision arthroplasty. Mencia et al. opted for single-stage partial exchange arthroplasty, preserving the well-cemented femoral stem [18]. In our and Athanasiou et al.’s [19] approach, a spacer was utilized, while Vergison et al. did not employ one during the first stage [17]. Kawakami et al. conducted surgical debridement with implant retention and antibiotic therapy [20], whereas Ramanathan and Ayoade solely administered antibiotic therapy without intra-articular prosthesis debridement or removal [21]. Mencia et al. utilized the Oxford Hip Score, yielding a score of 46 after post-single exchanged arthroplasty [18]. In our investigation, employing the HHS, we observed scores of 9 and 80 before and after two-stage revision arthroplasty. Another study documented an improvement in mean HHS from 52 points before spacer insertion to 70 points at 15 years after reimplantation [23]. Our study, alongside those of Vergison et al. [17], Athanasiou et al. [19], Mencia et al. [18], Kawakami et al. [20], and Ramanathan and Ayoade [21], demonstrated positive clinical outcomes.
PJIs have a significant clinical and economic impact, especially in older populations having TJA. This case highlights the complexities of PJI and its devastating impact on patients’ lives. Our patient’s experience, underscored by recurrent infections and limited treatment options due to socioeconomic constraints, highlights the need of accessible and affordable healthcare solutions. Despite the delay, the patient’s functional status and quality of life improved dramatically following the successful two-stage revision arthroplasty. Further research and healthcare initiatives are needed to address the complexity of PJI management and improve outcomes for those affected.
Early identification and prompt management of PJI, including appropriate antibiotic therapy and surgical intervention, are crucial for achieving favorable clinical outcomes and minimizing the burden on patients and healthcare systems.
References
- 1.Ong KL, Kurtz SM, Lau E, Bozic KJ, Berry DJ, Parvizi J. Prosthetic joint infection risk after total hip arthroplasty in the medicare population. J Arthroplasty 2009;24 6 Suppl:105-9. [Google Scholar]
- 2.Ren X, Ling L, Qi L, Liu Z, Zhang W, Yang Z, et al. Patients’ risk factors for periprosthetic joint infection in primary total hip arthroplasty: A meta-analysis of 40 studies. BMC Musculoskelet Disord 2021;22:776. [Google Scholar]
- 3.Izakovicova P, Borens O, Trampuz A. Periprosthetic joint infection: Current concepts and outlook. EFORT Open Rev 2019;4:482-94. [Google Scholar]
- 4.Peng HM, Zhou ZK, Wang F, Yan SG, Xu P, Shang XF, et al. Microbiology of periprosthetic hip and knee infections in surgically revised cases from 34 centers in mainland China. Infect Drug Resist 2021;14:2411-8. [Google Scholar]
- 5.Springer BD, Cahue S, Etkin CD, Lewallen DG, McGrory BJ. Infection burden in total hip and knee arthroplasties: An international registry-based perspective. Arthroplast Today 2017;3:137-40. [Google Scholar]
- 6.Lee QJ, Mak WP, Wong YC. Risk factors for periprosthetic joint infection in total knee arthroplasty. J Orthop Surg (Hong Kong) 2015;23:282-6. [Google Scholar]
- 7.Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: The current knowledge: AAOS exhibit selection. J Bone Joint Surg Am 2012;94:e104. [Google Scholar]
- 8.Ayoade F, Li DD, Mabrouk A, Todd JR. Periprosthetic Joint Infection. Florida: StatPearls Publishing; 2023 [Google Scholar]
- 9.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med 2004;351:1645-54. [Google Scholar]
- 10.Aggarwal VK, Rasouli MR, Parvizi J. Periprosthetic joint infection: Current concept. Indian J Orthop 2013;47:10-7. [Google Scholar]
- 11.Eka A, Chen AF. Patient-related medical risk factors for periprosthetic joint infection of the hip and knee. Ann Transl Med 2015;3:233. [Google Scholar]
- 12.Christensen DD, Moschetti WE, Brown MG, Lucas AP, Jevsevar DS, Fillingham YA. Dartmouth hitchcock medical center. Perioperative antibiotic prophylaxis: Single and 24-hour antibiotic dosages are equally effective at preventing periprosthetic joint infection in total joint arthroplasty. J Arthroplasty 2021;36:S308-13. [Google Scholar]
- 13.McMaster Arthroplasty Collaborative (MAC). Risk factors for periprosthetic joint infection following primary total hip arthroplasty: A 15-year, population-based cohort study. J Bone Joint Surg Am 2020;102:503-9. [Google Scholar]
- 14.Patel R. Periprosthetic joint infection. N Engl J Med 2023;388:251-62. [Google Scholar]
- 15.Parvizi J, Tan TL, Goswami K, Higuera C, Della Valle C, Chen AF, et al. The 2018 definition of periprosthetic hip and knee infection: An evidence-based and validated criteria. J Arthroplasty 2018;33:1309-14.e2. [Google Scholar]
- 16.Matthews PC, Berendt AR, McNally MA, Byren I. Diagnosis and management of prosthetic joint infection. BMJ 2009;338:b1773. [Google Scholar]
- 17.Vergison L, Schepens A, Liekens K, De Kesel R, Van der Bracht H, Victor J. Periprosthetic joint infection of a total hip arthroplasty with Candida parapsilosis. Int J Surg Case Rep 2020;69:72-5. [Google Scholar]
- 18.Mencia MM, Cawich SO, Sandiford N. Partial single stage exchange arthroplasty with retention of a well fixed cemented femoral stem for the treatment of culture negative infection in a bipolar hemiarthroplasty: A case report. Geriatr Orthop Surg Rehabil 2021;12:1-5. [Google Scholar]
- 19.Athanasiou V, Leonidou L, Lekkou A, Antzoulas P, Solou K, Diamantakis G, et al. Treatment of prosthetic joint infection due to Listeria monocytogenes. A comprehensive literature review and a case of total hip arthroplasty infection. Arthroplast Today 2022;13:48-54. [Google Scholar]
- 20.Kawakami S, Iwata K, Shimamura M, Mashiba T, Yokota K, Negayama K, et al. Prosthetic joint infection after total hip arthroplasty caused by Sneathia sanguinegens: A case report (CARE-complaint). Medicine (Baltimore) 2020;99:e22494. [Google Scholar]
- 21.Ramanathan M, Ayoade F. A case of Mycobacterium fortuitum prosthetic joint infection successfully treated medically without prosthesis explantation or joint debridement. BMJ Case Rep 2021;14:e243675. [Google Scholar]
- 22.Prado IP, Kim BI, Schwartz A, Wixted CM, Polascik B, Hendershot E, et al. 980. Enterobacter: An early-onset pathogen of prosthetic hip and knee infections with poor prognoses. Open Forum Infect Dis 2022;9 Suppl 2:492-822. [Google Scholar]
- 23.Petis SM, Abdel MP, Perry KI, Mabry TM, Hanssen AD, Berry DJ. Long-term results of a 2-stage exchange protocol for periprosthetic joint infection following total hip arthroplasty in 164 hips. J Bone Joint Surg Am 2019;101:74-84. [Google Scholar]