Compartment syndrome is typically treated with fasciotomies. There are case reports of paraspinal compartment syndrome treated conservatively for a multitude of reasons. This case series reviews the cases of conservatively treated paraspinal compartment syndrome case reports.
Dr. Mark LaGreca, Department of Orthopedic Surgery, Philadelphia College of Osteopathic Medicine, Philadelphia, United States of America. E-mail: ML194690@pcom.edu
Introduction: This review of case series and case reports explores conservative management strategies for paraspinal compartment syndrome (PCS), a rare clinical condition. Extremity compartment syndrome has been shown to be managed most effectively with emergent surgical release of the fascial compartment. Given the rarity of PCS and the paucity of research in the literature, some authors have suggested the possibility of conservative treatment. There has been no study to date that has specifically investigated the cases of non-operative management of PCS.
Materials and Methods: There are 16 case reports in the literature with 22 cases of PCS treated conservatively. The authors reviewed these cases, specifically viewing the clinical courses, why the decision was made to manage conservatively, and the reported outcomes.
Results: The etiology of PCS varied, with weightlifting being the primary cause in 11 out of 22 cases, followed by strenuous sporting events and postsurgical complications. All patients in this review were male, aged between 18 and 61 years old. Acute presentations exhibited severe back pain, rigid paraspinal musculature, and subjective paraspinal paresthesias. Magnetic Resonance Imaging findings of the spine revealed profound bilateral symmetric intramuscular edema. Among the cases, 8 explicitly reported a return to normal function, while 8 continued to experience symptoms related to the initial injury. Nine cases chose conservative measures primarily because of delayed presentation, seven instances reported successful outcomes with conservative measures; one case cited concerns about infection risk.
Discussion: The probability of underreporting related to PCS may result in a substantial number of cases being omitted from medical literature. Pathologically, PCS is characterized by increased intra-compartmental pressure, triggering rhabdomyolysis due to significant soft tissue damage. Emergent surgical intervention is the treatment of choice for any compartment syndrome; however, conservative management of these cases has shown satisfactory clinical outcomes. Hyperbaric oxygen therapy emerges as a potential adjunctive treatment to enhance tissue viability, though its efficacy and accessibility warrant further investigation in the context of PCS management.
Conclusion: Early recognition and treatment of PCS are critical in preventing chronic pain and permanent complications. Given the limitations identified in non-operative management, further research is imperative to optimize treatment strategies.
Keywords: Paraspinal compartment syndrome, conservative management, back pain.
Paraspinal compartment syndrome (PCS), initially identified by Carr et al. in 1985, has garnered attention through a small number of submitted case reports and case series over the years [1]. This syndrome manifests due to edema within the paravertebral myofascial compartment from a variety of causes, including exertion and post-operative cases [2]. Elevated intra-compartmental pressure (ICP) resulting from increased compartment volume can compromise the blood supply to the muscles, which may result in ischemia and subsequent muscle necrosis. A spectrum of perspectives exists on the management of this syndrome, leading to varied treatment modalities. The primary objective of this study was to review the literature on PCS, with the overarching goal of delineating conservative management approaches for this uncommon clinical entity.
A comprehensive literature search was performed using PubMed, MEDLINE, and Cochrane Library electronic databases. The search term “PCS” was used and appeared in the title, abstract, or keyword fields. The original search yielded 56 results. Articles were then examined for inclusion of non-operative management. We reviewed the reference lists of previously conducted systematic reviews to identify additional pertinent literature. The references cited in the identified studies were cross-checked to ensure the inclusion of any relevant material overlooked in the initial search. The chosen studies comprised original articles featuring one or more case reports detailing non-operative treatments for lumbar PCS. This systematic approach led to the identification of 22 non-operative cases derived from 16 distinct studies, all of which were incorporated into our comprehensive review (Fig. 1). The authors recorded information such as patient demographics, laboratory values, descriptions of imaging findings, compartment measurements, reasons for undergoing non-operative treatment, and reported clinical outcomes.
The causative etiology of the cases in our research varied, with weightlifting identified as the explicit cause in 12/22 cases (Table 1). Following this, incidents related to strenuous sporting events constituted 5/22 cases. Within this category, downhill skiing was a factor in 2/22 cases. These additional sporting events included a range of activities: Rowing, CrossFit exercises, and basketball-related activities. Postsurgical situations contributed to 5/22 cases, with abdominal vascular surgery contributing to 2/22 of instances, ankle surgery 1/22, aortoiliac bypass surgery 1/22, and gastric bypass surgery 1/22. The remaining and final case was from acute lower back pain (LBP) that woke the patient up from sleep.
All of the patients in this review were male, with ages ranging from 18 to 61 years old. Acute cases of PCS were most commonly associated with vigorous exercise, including weightlifting, specifically squatting or deadlifting (15/17 acute cases). The time span from the initiating event to the onset of acute lumbar compartment syndrome varied widely, ranging from minutes to hours. A majority (16/22) sought medical attention within the initial 36 h. However, notable outliers, such as cases in Saadat and Rezania and Fitch et al . presented with 7 years of chronic LBP postoperatively. 4/5 chronic cases were associated with surgery. The last chronic case was a patient with 2 years of LBP exacerbated by strenuous activity, namely downhill skiing. Acute presentations presented some form or combination of the following: unrelenting back pain, rigid paraspinal musculature, and subjective paraspinal paresthesias (14/17 acute cases). All acute cases were associated with elevated levels of creatine phosphokinase (average 35,333 U/L in those tested). Myoglobinuria was also noted across the identified case studies. Elevated liver enzymes, such as aspartate aminotransferase (AST) averaging 619 U/L in two cases (normal range <40 U/L), alanine aminotransferase (ALT) averaging 208 U/L in four cases (normal range <36 U/L), and lactate dehydrogenase averaging 2260.5 U/L in two cases (normal range <225 U/L), were observed. One case study noted the abuse of isotretinoin, cocaine, and testosterone by the patient. Acute presentations had magnetic resonance imaging (MRI) ordered at some point during the patient’s hospital stay. Bilateral symmetric intra-muscular edema affecting paraspinal muscles from T12 down to the sacrum is a consistent finding in these case reports (18/22). The MRI results in nearly every case were described as “extensive edema” or “myonecrosis in the paraspinal musculature”. Imaging also captured complications post-surgery, including areas of scarring and abnormal signals within paraspinal muscles. Patients with chronic lumbar PCS presented with exaggerated low back pain during exertion but were asymptomatic at rest, maintaining a normal range of spinal motion. MRIs in the chronic cases revealed fluid and fat infiltration of paraspinal musculature. In the overall clinical picture of acute paravertebral compartment syndrome, patients frequently exhibited severe back pain and rigid paraspinal musculature as the primary symptoms and exam findings. Laboratory values exhibited rhabdomyolysis often requiring interventions such as crystalloid fluid infusions and analgesics. Among cases specifying conservative approaches (22 in total), 9 cited delayed presentation and diagnosis as the rationale for conservative management. Conservative treatments yielded relief to a degree where surgical intervention was deemed potentially detrimental. Other considerations for non-operative management included elevated infection risk and low measured compartment pressure due to delayed presentation. In eight out of the 22 non-surgically treated cases, patients explicitly reported a return to normal, while eight continued to experience symptoms related to the initial insult. The remaining six did not specify the patients’ return-to-normal status. Most case reports noted a gradual improvement in pain, allowing a resumption of physical activities, though limitations persisted in more vigorous activities. Recovery times varied, with patients achieving a sense of full recovery spanning from 1 to 2 weeks to 1 year.
Paralumbar compartment syndrome was first reported as a possible diagnosis by Peck et al. in 1981. It was not until 4 years later that Carr et al. published a case report on the first diagnosed paralumbar compartment syndrome in 1985. There have been few case reports and case series written since; some were treated with fasciotomies and some were treated conservatively. However, the uncommon occurrence of PCS in clinical practice may hinder consistent management. It is essential to acknowledge the lack of consensus surrounding the management of PCS, leaving case reports and case series as the sole point of reference for comparison. Hence, clinicians face the challenge of recognizing atypical manifestations of uncommon conditions such as PCS. This necessitates a thorough understanding of the anatomy, pathology, diagnostic nuances, and treatment options specific to PCS, enabling clinicians to promptly and accurately manage the condition.
Anatomical considerations
Understanding the function of the thoracolumbar fascia (TLF) is pertinent in the context of PCS. Historically, the TLF was thought to primarily serve as the origin for abdominal wall muscles [3]. However, a study by Tesh et al. has shown that the arrangement of the fibers in the fascia suggests that the internal oblique and transversus abdominis muscles arise principally from the middle layer of the TLF with only a restricted origin from the posterior layer. Their study highlights the TLF’s main function as forming a compartment around the lower lumbar and sacral paraspinal muscles, contributing to the rise of ICP during erector spinae muscle contraction. Cadaver dissections by Carr et al. and Willard et al. support these findings, characterizing the TLF by well-defined fascial sheaths and attachments to surrounding bony structures, underscoring its critical function in isolating the paraspinal muscles [1, 4]. The irregular arrangement of collagen fibers in the TLF imparts an unyielding and non-distensible quality, allowing it to function as packing tissue and resist tensional forces universally [4]. In instances of PCS, patients commonly exhibit back pain following trauma, with the syndrome often manifesting in the context of sporting events. This is particularly evident during high-intensity exercises such as heavy back squats, downhill skiing, sprints, and rowing, which impose substantial compression and loading on the lumbar paraspinal muscles, resulting in heightened compartment pressure. Recorded pressures in all cases surpassed thresholds recommended by Songcharoen et al. 5 mmHg for normal, healthy paravertebral compartmental pressure. Resting ICP in healthy individuals typically ranges from 3 to 7.95 mmHg, with transient increases up to 25 mmHg during exercise, returning to preexisting ICP within 1–6 min[5]. Typically, pulse pressure, also known as delta pressure, is used as a quantitative indicator for diagnosing compartment syndrome. This metric, calculated by subtracting intramuscular pressure from diastolic blood pressure, offers a threshold of 30 mm Hg or below to signify inadequate perfusion to the extremity [6]. Delta pressure may be used in conjunction with other clinical symptoms of compartment syndrome.
Pathology
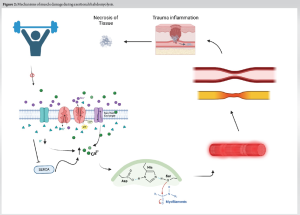
Rhabdomyolysis, manifesting as a secondary consequence of compartment syndrome, is marked by an increase in serum creatine kinase. Better and Stein proposed that exertional and metabolic rhabdomyolysis in humans result in impaired sarcolemma sodium-potassium-adenosine triphosphatase activity in damaged muscles [7]. This impairment contributes to the disruption of myofibrils and muscle damage through the activation of neutral proteases due to an increase in cytosolic-free calcium [7]. In addition, in injured tissue, the breakdown of crucial energy-dependent transcellular pump systems, such as the Na/K-ATPase and Ca2+ATPase pumps, vital for myocyte integrity, can result in muscle cell swelling [8]. This swelling elevates intramuscular pressure within the injured muscle, exceeding arteriolar-perfusion pressure and leading to weakened contraction of the muscle fibers and myoneural ischemic damage in some cases. Fig. 2 is a demonstration of this pathway.
Furthermore, the increase in pressure within the compartment may surpass capillary hydrostatic pressure, causing vascular stasis and prompting a shift toward anaerobic metabolism. This, in turn, induces oxygen debt within skeletal muscles, leading to increased capillary permeability and initiating inflammatory cascades [7]. Consequently, a significant rise in ICP ensues, causing additional muscle damage, severe impairment of local circulation, and neuromuscular function [2, 9]. Blood flow to the paraspinal musculature is managed by the dorsal branches of parietal arteries, known as intercostal and lumbar arteries. These arteries derive from the abdominal aorta [1]. The innervation of paraspinal muscles is facilitated by spinal nerves that emerge bilaterally through the intervertebral foramen between adjacent vertebrae. These nerves split into dorsal and ventral rami below this foramen [10]. Lower back numbness and par esthesias occur due to dorsal rami and cutaneous branches of the cluneal nerve, which innervate the lumbar skin, muscles, and fasciae of the lumbar region [10]. Early signs of PCS may include sensory loss in nerve distribution, as peripheral nerves exhibit greater sensitivity to ischemia than muscle. Previous canine studies by Rorabeck and Macnab demonstrated that nerve conduction velocity significantly decreased at 30 mm Hg compartment pressure after 8 h , with a complete block occurring at 50 mm Hg [11]. Early detection and treatment will prevent irreversible damage to nerve tissues in the paraspinal area. Out of the 22 patients examined in the case studies, 8 had reported experiencing par esthesias in the lumbar region. In this review of conservatively treated cases, eight cases explicitly reported a return to normal function, while eight continued to experience symptoms related to the initial injury, and six cases did not specify the patients’ recovery status. However, contrasting outcomes were observed in existing review studies advocating for surgical decompression, where the majority of PCS patients (19 out of 20) achieved full recovery and resumed normal activities following fasciotomy [12]. Given these findings, timely surgical decompression emerges as crucial in alleviating paraspinal compartment pressures. This approach facilitates tissue reperfusion and prevents further ischemic muscle damage by restoring blood flow to the affected areas.
PCS recognition
The differential for LBP is vast. This includes but is not limited to renal colic, kidney stones, osteomyelitis, epidural hematoma/abscess, degenerative arthritis of the lumbar spine, spondylolisthesis, intervertebral disk bulging or herniation, and facet joint arthropathy. PCS is diagnosed through various subjective and objective clinical features. In the acute setting, these patients tend to have a reported recent history of physical exertion including weightlifting or sporting events. On physical examination, the clinician may find severe pain disproportionate to the reported history of present illness exacerbated by passive muscle stretch, paraspinal swelling, extreme paraspinal tenderness to palpation, and subjective paresthesias in the lumbar spine and sacral regions. Objective measurements to aid in the diagnosis include measurement of intramuscular pressure, and lab values such as elevated creatine kinase, ALT, and AST. Imaging can also help aid in the diagnosis such as MRI findings of significant paraspinal edema contribute to confirmation. Missed diagnosis of PCS can lead to severe sequelae. This sequela is exemplified in two cases reported by Haig et al. The first case was a 57-year-old male who underwent an aortoiliac bypass procedure, developed severe back pain 2 days later, and was diagnosed with a mild herniated disc. Reviewing the MRI obtained 2 days post-operatively, months later, revealed significant edema in the paraspinal musculature; this patient reported pain with standing, sitting, and lifting. A repeat MRI performed on this patient 11 months later demonstrated significant fatty atrophy of the paraspinal musculature. The other case described by Haig et al. presented a 34-year-old male after a gastric bypass procedure, 1 year post-operatively with complete loss of touch and pinprick sensation from T11 to the upper sacrum. Moreover, et al. detailed a case study featuring a 61-year-old male patient who endured 7 years of chronic back pain subsequent to abdominal vascular surgery [14]. The patient exhibited pain, tenderness, and sensory loss in the lumbar paraspinal muscle region. The authors confirmed their suspicion of missed paravertebral compartment syndrome through MRI. Given the rarity of this diagnosis and the limited information available regarding the clinical symptoms, physicians may overlook the diagnosis if they are not familiar with the relevant indicators. Timely diagnosis and consideration as a differential are essential to mitigate its debilitating functional consequences and ensure optimal patient outcomes.
Hyperbaric oxygen
The optimal treatment strategy for PCS remains an ongoing challenge within the medical field. Increased ICP acts as a catalyst for further harm and necrotic processes. Given the closed, non-communicating nature of these compartments, the primary method for pressure reduction involves surgical decompression through fasciotomy. However, not all researchers universally support performing early fasciotomy due to concerns about the risk of potential infections associated with the procedure [16]. Nevertheless, there is consensus among a majority of cases regarding the significance of fluid resuscitation in mitigating the progression of rhabdomyolysis. This recommendation aligns with clinical observations in humans, suggesting that administering intravenous fluids equivalent to the extracellular space can effectively infiltrate injured muscles within a relatively short time following the injury [7]. In addition to standard therapies such as aggressive fluid administration and analgesics, some authors have explored alternative treatments such as hyperbaric oxygen (HBO) therapy [17, 18]. Studies by et al. demonstrated successful pain management with HBO, possibly through mechanisms involving increased tissue oxygenation and vasoconstriction [19, 20]. Clinical trials have demonstrated that HBO therapy immediately increases oxygen delivery to ischemic tissue through hyperoxygenation [19]. In addition, hyperoxygenation induces direct vasoconstriction, potentially reducing edema in the paravertebral compartment by lowering the capillary transudation flow rate [19]. This effect is supported by research from Sullivan and Johnson, which shows that increased tissue PO2 levels diminish the autoregulatory dilation mechanism at all levels of the microcirculation in skeletal muscle, indicating the vasoconstrictive properties of hyperoxygenation [20]. However, the efficacy of HBO therapy is contingent upon adequate tissue perfusion, necessitating lowering of compartment pressure before its application. Furthermore, it is noteworthy that in the case studies of the two patients who received HBO therapy, back pain persisted during exertion, indicating that it may not completely alleviate symptoms and restore patients to their baseline condition. Thus, HBO therapy may hold promise as an adjunctive treatment following initial surgical management to enhance tissue viability in PCS. Moreover, the limited availability of HBO therapy facilities, particularly in major medical hubs such as Philadelphia, poses a challenge to the reliability and availability of this treatment. Ongoing research is essential for establishing comprehensive long-term outcome data on the efficacy of hyperbaric treatment as a supplementary therapy for PCS. List of all cases are listed in Table 1.
This study has several limitations. For one, this is a retrospective review of case reports and case series, so the data collected are limited to what is written by the authors. There were various absent clinical and laboratory data points. The authors of this case study review were also limited by the inconsistent follow-up for these patients and the outcome descriptions. The absence of reported outcomes in 5 out of 22 cases receiving conservative measures limits the ability to comprehensively assess the effectiveness of non-operative interventions. There were no objective or subjective patient-reported scales utilized when describing the patient’s post-hospitalization course.
This is the first review to investigate conservative treatments for PCS. PCS diagnosis is often overlooked due to its rarity and can be missed without specific clinical suspicion. Therefore, recognizing the significance of early diagnosis is crucial, as undiagnosed or delayed PCS can result in severe and chronic disability, affecting daily life activities. Patients managed conservatively experienced a return to baseline functions within a span of 1 week to 1 year, albeit often enduring chronic back pain during exertion. This is particularly concerning for athletes but potentially viable for non-athletes or chronic cases. Furthermore, surgical decompression in the acute phase may mitigate tissue damage. Further research is needed to enhance our understanding of PCS and guide the development of more effective therapeutic strategies in clinical practice.
PCS should be treated with fasciotomies in the acute setting. Most of those treated conservatively have persistent symptoms with exertion.
References
- 1.Carr D, Gilbertson L, Frymoyer J, Krag M, Pope M. Lumbar paraspinal compartment syndrome. A case report with physiologic and anatomic studies. Spine (Phila Pa 1976) 1985;10:816-20. [Google Scholar]
- 2.Nathan ST, Roberts CS, Deliberato D. Lumbar paraspinal compartment syndrome. Int Orthop 2012;36:1221-7. [Google Scholar]
- 3.Tesh KM, Dunn JS, Evans JH. The abdominal muscles and vertebral stability. Spine (Phila Pa 1976) 1987;12:501-8. [Google Scholar]
- 4.Willard FH, Vleeming A, Schuenke MD, Danneels L, Schleip R. The thoracolumbar fascia: Anatomy, function and clinical considerations. J Anat 2012;221:507-36. [Google Scholar]
- 5.Songcharoen P, Chotigavanich C, Thanapipatsiri S. Lumbar paraspinal compartment pressure in back muscle exercise. J Spinal Disord 1994;7:49-53. [Google Scholar]
- 6.Whitesides TE, Haney TC, Morimoto K, Harada H. Tissue pressure measurements as a determinant for the need of fasciotomy. Clin Orthop Relat Res 1975;113:43-51. [Google Scholar]
- 7.Better OS, Stein JH. Early management of shock and prophylaxis of acute renal failure in traumatic rhabdomyolysis. N Engl J Med 1990;322:825-9. [Google Scholar]
- 8.Vucicevic Z. Rhabdomyolysis and acute renal failure after gardening. Case Rep Emerg Med 2015;2015:174892. [Google Scholar]
- 9.Rogers ME, Lowe JA, Vanlandingham SC. Acute erector spinae compartment syndrome: Case report and review of diagnostic criteria. Injury 2014;45:813-5. [Google Scholar]
- 10.Creze M, Ghaouche J, Missenard G, Lazure T, Cluzel G, Devilder M, et al. Understanding a mass in the paraspinal region: An anatomical approach. Insights Imaging 2023;14:128. [Google Scholar]
- 11.Rorabeck CH, Macnab L. Anterior tibial-compartment syndrome complicating fractures of the shaft of the tibia. J Bone Joint Surg Am 1976;58:549-50. [Google Scholar]
- 12.Ilyas H, Fagan C, Roser F, Hebela NM. Lumbar paraspinal compartment syndrome: Case report and critical evaluation of the literature. Clin Spine Surg 2022;35:301-9. [Google Scholar]
- 13.Haig AJ, Hartigan AG, Quint D. Low back pain after nonspinal surgery: The characteristics of presumed lumbar paraspinal compartment syndrome. PM R 2009;1:383-8. [Google Scholar]
- 14.Wik L, Patterson JM, Oswald AE. Exertional paraspinal muscle rhabdomyolysis and compartment syndrome: A cause of back pain not to be missed. Clin Rheumatol 2010;29:803-805. [Google Scholar]
- 15.Ferreira J, Galle C, Aminian A, Michel P, Guyot S, De Wilde JP, et al. Lumbar paraspinal rhabdomyolysis and compartment syndrome after abdominal aortic aneurysm repair. J Vasc Surg 2003;37:198-201. [Google Scholar]
- 16.Ogoshi T, Yoshimiya M, Ichibakase H, Kimura T, Kameoka M, Yoshioka H, et al. Paravertebral compartment syndrome after exercise: A case report. J Med Case Rep 2020;14:208. [Google Scholar]
- 17.Allerton C, Gawthrope IC. Acute paraspinal compartment syndrome as an unusual cause of severe low back pain. Emerg Med Australas 2012;24:457-9. [Google Scholar]
- 18.Karam MD, Amendola A, Mendoza-Lattes S. Case report: Successful treatment of acute exertional paraspinal compartment syndrome with hyperbaric oxygen therapy. Iowa Orthop J 2010;30:188-90. [Google Scholar]
- 19.Eskes A, Vermeulen H, Lucas C, Ubbink DT. Hyperbaric oxygen therapy for treating acute surgical and traumatic wounds. Cochrane Database Syst Rev 2013;2013:CD008059. [Google Scholar]
- 20.Bouachour G, Cronier P, Gouello JP, Toulemonde JL, Talha A, Alquier P. Hyperbaric oxygen therapy in the management of crush injuries: A randomized double-blind placebo-controlled clinical trial. J Trauma 1996;41:333-9. [Google Scholar]
- 21.Saadat N, Rezania K. Postoperative lumbar paraspinal compartment syndrome. BMJ Case Rep 2021;14:4-7. [Google Scholar]
- 22.DiFazio FA, Barth RA, Frymoyer JW. Acute lumbar paraspinal compartment syndrome. A case report. J Bone Joint Surg Am 1991;73:1101-3. [Google Scholar]
- 23.Kanaya H, Enokida M, Tanishima S, Hayashi I, Tanida A, Nagashima H. Conservative treatment for lumbar compartment syndrome shows efficacy over 2-year follow-up: A case report and literature review. Arch Orthop Trauma Surg 2017;137:1233-8. [Google Scholar]
- 24.Hoyle A, Tang V, Baker A, Blades R. Acute paraspinal compartment syndrome as a rare cause of loin pain. Ann R Coll Surg Engl 2015;97:e11-2. [Google Scholar]
- 25.Chavez JM, Gonzalez PG. Suspected lumbar compartment syndrome: A rare cause of low back pain after strenuous exercise. Spine J 2013;13:1409-10. [Google Scholar]
- 26.Calvert N, Bhalla T, Twerenbold R. Acute exertional paraspinal compartment syndrome. ANZ J Surg 2012;82:564-5. [Google Scholar]
- 27.Anaya A, Plantmason L, Dhaliwal G. Clinical practice: Exercises in clinical reasoning back attack. J Gen Intern Med 2013;29:255-9. [Google Scholar]
- 28.Eichner ER, Schnebel B, Anderson S, Clugston JR, Hale MH, Michaudet C, et al. Acute lumbar paraspinal myonecrosis in football players with sickle cell trait: A case series. Med Sci Sports Exerc 2017;49:627-32. [Google Scholar]
- 29.Fitch DS, Leung D, Reish AG. Extensive lumbar paraspinal fluid/fat collections from lumbar paraspinal myonecrosis and presumed subsequent compartment syndrome: 7 years post onset. Spine J 2014;14:1077-8. [Google Scholar]