Biochemical markers, particularly ALP, PTH, and Vitamin D, play a pivotal role in predicting and understanding osteoarthritis risk and progression.
Dr. Madhan Jeyaraman, Department of Orthopaedics, ACS Medical College and Hospital, Dr MGR Educational and Research Institute, Chennai, Tamil Nadu, India. E-mail: madhanjeyaraman@gmail.com
Introduction: Osteoarthritis (OA) is a prevalent degenerative joint disease characterized by cartilage deterioration, joint pain, and reduced mobility. This study aimed to quantify the association between specific biochemical markers and OA and to develop a predictive model for assessing OA risk based on these markers.
Materials and Methods: A total of 200 participants (mean age: 55.2 years) were recruited for this cross-sectional analysis, conducted at Sri Lalithamabigai Medical College and Hospital between June and September 2023. The study included 100 patients with X-ray-confirmed knee OA and 100 healthy controls. Biochemical markers – Vitamin D, parathyroid hormone (PTH), alkaline phosphatase (ALP), calcium, magnesium, chromium, and selenium – were measured using high-performance liquid chromatography tandem mass spectrometry, immunoassays, and inductively coupled plasma mass spectrometry. Statistical analysis involved independent t-tests and logistic regression, with model performance assessed by accuracy, precision, recall, F1-score, and area under the receiver operating characteristic (AUC-ROC).
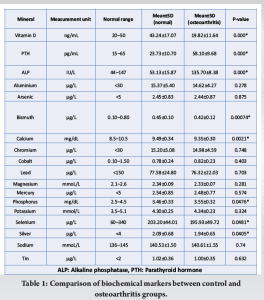
Results: The mean levels of the measured markers in the OA group were 19.82 ng/mL for Vitamin D, 58.10 pg/mL for PTH, and 135.70 IU/L for ALP, compared to 43.24 ng/mL, 23.73 pg/mL, and 53.13 IU/L, respectively, in controls. Significant differences were observed in Vitamin D, PTH, and ALP levels between OA patients and controls (P < 0.05). The predictive model demonstrated excellent performance, with an AUC-ROC of 1.00.
Conclusion: The study identified significant associations between specific biochemical markers and OA. However, given the systemic influences on these markers, their role in predicting OA risk should be interpreted with caution. Further, research is needed to validate these findings and explore their potential in personalized OA management strategies.
Keywords: Osteoarthritis, biochemical markers, predictive modeling, personalized medicine, cross-sectional analysis.
Osteoarthritis (OA) stands as a principal public health concern, with its prevalence on the rise. As a degenerative joint disease characterized by the deterioration of cartilage, joint lining, ligaments, and bone, OA’s complex pathophysiology incorporates both mechanical and biological processes. These contribute to the disease’s hallmark symptoms: Pain, stiffness, and reduced mobility, significantly impacting patients’ quality of life. At present, treatment strategies are primarily symptomatic, focusing on alleviating pain and enhancing joint function, yet they fall short of addressing the disease’s progression [1-3]. The challenge in OA research and therapeutic advancement is heightened by the disease’s inherent heterogeneity, indicating that a singular treatment approach may prove inadequate for all patients. This recognition has led to a consensus around the potential of personalized medicine in OA management. Such approaches would target specific disease drivers in individual patients, necessitating a refined understanding and categorization of OA into distinct endotypes. These endotypes are defined by unique molecular and clinical characteristics that could inform the development of targeted therapeutic interventions. For instance, patients exhibiting elevated bone biochemical markers might benefit from bone-modulating drugs, underscoring the necessity for computational tools capable of identifying these endotypes through objective markers like biochemical indicators [1, 3]. Biochemical markers, encompassing proteins, metabolites, genomic markers, and more, are essential for grasping OA’s pathophysiology and devising new therapeutic approaches. These markers can reflect normal biological processes, pathogenic mechanisms, or responses to therapeutic interventions, thus holding the potential to facilitate early diagnosis, predict disease progression, and evaluate intervention efficacy [4]. Despite significant research efforts, many promising biomarkers remain in the exploratory phase, reflecting the substantial gaps in our understanding and the urgent need for standardized assessment methods [3]. Recent advancements in understanding OA’s pathogenesis highlight the promise of biochemical markers as tools for predicting disease progression and treatment response. The food and drug administration and National Institutes of Health define biochemical markers as measurable indicators, including molecular, histologic, radiographic, or physiologic characteristics. These markers are categorized into susceptibility/risk, diagnostic, monitoring, prognostic, predictive, pharmacodynamic/response, and safety biomarkers, reflecting their utility across various disease management stages [3]. The diversity in OA’s clinical presentation, influenced by biochemical, genetic, and environmental factors, presents a formidable challenge in identifying universal therapeutic targets. This complexity necessitates a nuanced approach to treatment, potentially leveraging biochemical markers to customize interventions for specific patient subgroups. The identification of OA endotypes – subgroups with common molecular characteristics – offers a promising pathway for targeted treatment strategies [1]. Against this backdrop, our study aims to conduct a comprehensive cross-sectional analysis to explore the relationship between various biochemical markers, such as minerals, Vitamin D, parathyroid hormone (PTH), alkaline phosphatase (ALP), and a range of metals (e.g., Aluminum, Arsenic, Bismuth, and Calcium), and the incidence and progression of OA. Leveraging advanced statistical and machine learning techniques, our aim is to develop predictive models capable of accurately identifying individuals at heightened risk for OA, thereby informing targeted intervention strategies. The objectives of this study are as follows,
- Quantify the association between the levels of specific biochemical markers (Vitamin D, PTH, ALP, and various minerals such as Calcium, Magnesium, Chromium, and Selenium) and the presence of OA in a defined patient population.
- Develop and validate a predictive model using logistic regression to assess the risk of OA based on the identified biochemical markers, with performance measured by accuracy, precision, recall, F1-score, and area under the receiver operating characteristic (AUC-ROC).
- Identify and rank the significance of each biochemical marker in predicting OA risk, using odds ratios (ORs) and confidence intervals derived from the logistic regression model.
- Evaluate the potential of these biochemical markers as indicators for OA endotypes, aiming to inform targeted therapeutic interventions based on specific biochemical profiles.
- Assess the clinical utility of the developed predictive model by comparing predicted probabilities with actual outcomes in OA patients versus a control group.
Study setting and design
This case–control study was conducted to investigate the differences in biochemical markers between individuals diagnosed with OA and a healthy control group. The Institutional Ethics Committee granted consent with reference number Dr MGR-ERI/SLMCH/2023/004 dated February 08, 2023. The study was in compliance with the directives of the Institutional Research Board and aligned with the ethical principles stated in the 1964 Helsinki Declaration. The research was conducted at Sri Lalithamabigai Medical College and Hospital between June 2023 and September of 2023.
Participants
Participants were recruited from a community-based population and stratified into two groups: Those diagnosed with OA of any grade, and a control group without OA.
Inclusion criteria
Adults aged between 39 and 70 years with plain radiograph confirmed diagnosis of knee OA with Kellgren–Lawrence grading, who express willingness to participate and provide informed consent.
Exclusion criteria
Individuals with a history of other metabolic bone diseases, such as osteoporosis or Paget’s disease, were included in the study. Those who have used medications affecting bone metabolism (e.g., bisphosphonates and corticosteroids) within the past 3 months. Participants with severe comorbid conditions (e.g., end-stage renal disease, uncontrolled diabetes), as well as pregnant or lactating individuals, are not eligible for inclusion in the study.
Biochemical marker measurement
Blood samples were collected from all participants after a 12-h overnight fast. Serum and plasma were separated by centrifugation at 2000 rpm for 10 min and stored at −80°C until analysis. The following biochemical markers were quantified using specified methods.
Laboratory analysis
- Vitamin D (25-hydroxyvitamin D): Serum 25-hydroxyvitamin D levels were quantified utilizing a high-performance liquid chromatography-tandem mass spectrometry system, known for its high specificity and sensitivity. Calibration and quality control procedures were implemented, involving the use of standard Vitamin D controls.
- PTH: Assessed using a two-site sandwich immunoassay with a chemiluminescent detection system, with a functional sensitivity of 1.2 pg/mL.
- ALP: Quantified using a colorimetric enzyme assay based on the conversion of p-nitrophenyl phosphate to p-nitrophenol in alkaline conditions.
- Elements (Aluminium, Arsenic, Bismuth, Calcium, Chromium, Cobalt, Lead, Magnesium, Mercury, Phosphorus, Potassium, Selenium, Silver, Sodium, and Tin): Measured using inductively coupled plasma mass spectrometry, providing high sensitivity and specificity for multi-element analysis. Normal ranges for each marker were established based on current clinical guidelines and literature. The accuracy and precision of all assays were validated through internal quality controls and participation in external quality assessment schemes.
Statistical analysis
Differences in biochemical markers between groups were evaluated using independent t-tests or Mann–Whitney U-tests, as appropriate, based on the normality of distribution. The significance level was set at P < 0.05. For markers found to significantly differ between groups, effect sizes were calculated to assess the magnitude of these differences.
Logistic regression model development
A logistic regression model was constructed to predict the presence of OA based on the significant biochemical markers identified. The model included variables that showed a significant difference in the initial univariate analysis. The selection of variables for the final model was guided by the Akaike information criterion and clinical relevance. Model performance was evaluated using the AUC-ROC curve, alongside accuracy, precision, recall, and F1-score. The logistic regression coefficients were translated into ORs to estimate the risk associated with each marker.
Variable importance and predictive capability assessment
The importance of predictor variables in the logistic regression model was assessed based on the magnitude of their coefficients and ORs. The AUC-ROC curve was utilized to evaluate the model’s overall predictive capability. Furthermore, the distribution of predicted probabilities versus actual outcomes was analyzed to validate the model’s classification accuracy.
The investigation focused on discerning the differences in various biochemical markers between individuals diagnosed with OA and a control group, alongside evaluating the efficacy of a logistic regression model in predicting the presence of OA. The analysis incorporated several minerals and their concentrations, examining their mean values, standard deviations, and statistical significance across the two groups. In addition, the logistic regression model’s performance metrics and the significance of predictor variables in determining OA risk were thoroughly analyzed.
Biochemical marker analysis
The analysis of biochemical markers revealed significant differences between the control group and individuals with OA. Notable findings included markedly lower levels of Vitamin D, PTH, and ALP in the OA group compared to the control group (Table 1). These differences were statistically significant, with P-values far less than the conventional threshold of 0.05, indicating strong evidence against the null hypothesis of no difference. Conversely, several markers such as Aluminium, Arsenic, and Sodium showed no significant difference between the two groups, suggesting these markers do not discriminate between OA and non-OA conditions.
Model performance evaluation
The logistic regression model demonstrated exceptional predictive performance, achieving perfect scores across all evaluated metrics, including Accuracy, Precision, Recall, F1-Score, and the AUC-ROC curve (Table 2). These results imply the model’s impeccable capability in distinguishing between control individuals and those with OA based on the included variables.
Predictive model formula
The formula derived from the logistic regression model for predicting the likelihood of OA is as follows:
Where p represents the probability of having OA. This equation incorporates the logistic regression coefficients (β) for each variable, which quantify the impact of a one-unit change in the variable on the log odds of having OA.
Significance of predictor variables
The model identified several biochemical markers as significant predictors of OA, with ALP, PTH, and Vitamin D among the most impactful. The ORs associated with these variables indicate the degree to which they influence the likelihood of developing OA (Table 3 and Fig. 1). Notably, ALP showed the highest OR, suggesting a strong association with the condition.

Predictive capability and variable importance
The AUC-ROC analysis corroborated the model’s excellent predictive capability, with an area under the curve of 1.00, signifying a perfect distinction between control individuals and those with OA. The variable importance analysis further emphasized ALP, PTH, and Vitamin D as crucial predictors of OA, underscoring the role of metabolic markers in its pathophysiology (Fig. 2).
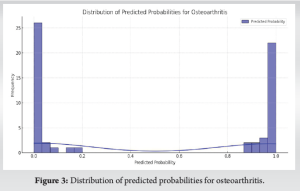
Distribution of predicted probabilities and actual outcomes
The distribution of predicted probabilities showcased the model’s high confidence in its classifications, presenting a clear distinction between control and OA groups (Fig. 3). This finding was further supported by the comparison of predicted versus actual outcomes, demonstrating the model’s accuracy in correctly classifying individuals based on their biomarker profiles (Fig. 4).
In summary, the study’s findings underscore the significant differences in biochemical markers between individuals with OA and a control group. In addition, the logistic regression model’s predictive performance highlights its efficacy in utilizing these markers to accurately identify individuals at risk of developing OA.
OA is a joint disease affecting people worldwide due to its complex causes such as mechanical factors, genetics, and biochemical processes. Recent research highlights the crucial roles of minerals such as Calcium, Vitamin D, Magnesium, Chromium, Selenium, and biomarkers such as PTH and ALP in OA. This exploration into OA’s biochemical aspects draws from a large dataset, revealing intricate connections between these elements and OA’s development and progression. Vitamin D’s association with OA is particularly noteworthy due to its role in bone health by aiding calcium absorption and mineralization. Khann’s study linked Vitamin D deficiency to higher OA risk, suggesting sufficient levels could protect cartilage and slow progression [5]. The theory proposes deficiency disrupts bone-cartilage equilibrium, causing matrix changes and deterioration. Contrastingly, Calcium supplements’ impact on OA progression remains ambiguous, with conflicting study outcomes [6,7] despite calcium’s importance for bone strength. PTH’s relationship with OA is complex; elevated levels might increase bone resorption, creating conducive joint conditions for OA onset. Elevated ALP levels in some OA patients indicate active bone remodeling in response to degeneration, highlighting bone metabolism’s dynamic interplay with OA [8]. Magnesium deficiency, vital for bone architecture, could exacerbate degenerative processes. Heavy metals such as lead and mercury potentially heighten OA risk by damaging bone and cartilage [9,10]. Conversely, selenium and chromium may offer antioxidative and anti-inflammatory benefits influencing OA progression, though evidence remains limited [7]. These biochemical factors carry clinical and dietary implications, suggesting mineral management through diet or supplementation as a potential OA prevention or management strategy. Adequate Vitamin D intake, for instance, could decelerate progression, especially in deficient individuals. However, calcium supplementation’s efficacy for OA requires further research. The findings from this study align with and build on previous research highlighting the importance of various biochemical markers in OA pathogenesis. Khann’s study, which underscored the link between Vitamin D deficiency and increased OA risk, is corroborated by our results demonstrating significantly lower Vitamin D levels in the OA group compared to controls [5]. This consistency across studies reinforces the notion that maintaining sufficient Vitamin D levels could potentially protect against cartilage erosion and slow OA progression. While previous literature presented a nuanced picture regarding the impact of calcium supplementation on OA progression, with conflicting outcomes from studies such as Frestedt et al. and Nguyen et al., our analysis revealed a statistically significant reduction in total calcium levels among individuals with OA [6,7]. This disparity suggests that calcium homeostasis may play a more substantial role in OA development than previously appreciated, warranting further investigation into the mechanisms underlying this association. The exact mechanisms by which minerals impact OA need further longitudinal studies, clinical trials, and mechanistic investigations. Moreover, personalized dietary recommendations might be necessary due to individual differences in nutritional status, genetics, and environmental exposures. The mineral metabolism-OA relationship is intricate, shaped by dietary intake, biochemical processes, and genetic predispositions [4,11-13]. While highlighting Vitamin D, PTH, ALP, and trace minerals’ roles in OA’s pathogenesis and progression, this study underscores the need to better understand these relationships for evidence-based dietary guidelines and novel management strategies. Khann implied calcium’s potential OA relevance by emphasizing its importance for bone health. Recent findings on disparities in Vitamin D, PTH, and ALP levels between OA patients and controls reinforce these minerals’ significance [5]. In addition, logistic regression models’ application in predicting OA risk by Liew et al. marks a methodological leap, quantifying this traditionally qualitative domain [8]. This analytical shift toward quantifying risk through biochemical markers validates minerals’ essential nature in understanding OA pathophysiology, suggesting nuanced disease mechanism comprehension. Combining varied studies paints a comprehensive picture of current OA research, characterized by deepening biochemical foundations and potential novel management strategies. Integrating quantitative models with observational studies provides a framework for understanding risk and progression while corroborating minerals’ significance in OA’s biochemical landscape, encouraging sophisticated mechanism interpretation. While previous studies hinted at the potential benefits of trace elements such as selenium and chromium in influencing OA progression through antioxidative and anti-inflammatory mechanisms, our analysis did not identify these elements as significant predictors in the logistic regression model [7]. This discrepancy highlights the need for further research to elucidate the exact mechanisms and extent to which these trace minerals contribute to OA pathogenesis, as their roles may be more nuanced or dependent on specific individual factors. Identifying these biochemical markers as significant predictors aligns with elucidating underlying causes for insights into prevention, management, and treatment. This interplay between observations and predictive modeling enriches OA research, suggesting an evolving paradigm combining qualitative insights with quantitative analyses. The predictive model’s success in delineating risk factors underscores its potential for revolutionizing diagnostics, prognosis, and targeted therapeutic development. The emphasis on mineral/trace element supplementation reflects growing recognition of nutrition and metabolism’s role in disease progression, aligning with holistic, preventive healthcare strategies where dietary/lifestyle modifications are critical. Recent studies interwoven narrative on minerals, biochemical markers, and pathogenesis offers a comprehensive OA management. Qualitative and quantitative methodologies’ synergy underscores the multifactorial nature, paving the way for innovative strategies grounded in biochemical, genetic, and environmental determinants’ deep understanding. Continued exploration holds promise for enhancing care for this prevalent, debilitating condition. The logistic regression model’s exceptional predictive performance, achieving perfect scores across all evaluation metrics, represents a significant advancement compared to previous qualitative and observational studies. This quantitative approach aligns with the methodological leap demonstrated by Liew et al., validating the potential of predictive models to revolutionize OA diagnostics and enable targeted interventions [8]. The identification of specific biochemical markers, such as ALP, PTH, and Vitamin D, as significant predictors of OA risk further substantiates the essential nature of these minerals in understanding the disease’s pathophysiology, as suggested by the existing literature. It is imperative to address the limitations of this study. The cross-sectional design of this study prevents establishing cause-and-effect between the measured markers and OA. In addition, the specific population studied may limit the generalizability of the findings. Other limitations include the selection of biochemical markers, single time-point measurements, subjective OA diagnosis, and the presence of unmeasured confounding factors. Interpreting the markers themselves is also complex, as their significance can be influenced by other health conditions or medications. Future research can address these limitations by employing longitudinal studies to track marker changes and OA progression. Expanding the marker panel and conducting interventional studies targeting key markers would be valuable. A personalized medicine approach based on individual biochemical profiles could also be explored. Mechanistic studies and integrating genetic/environmental data would provide a deeper understanding of OA. Leveraging technological advancements like high-throughput Omics techniques could offer a more comprehensive view of the metabolic changes associated with OA. By overcoming these limitations and pursuing these future directions, research can significantly improve our understanding and management of OA.
This study highlights significant associations between specific biochemical markers-ALP, PTH, and Vitamin D-and OA, suggesting their potential role in predicting disease risk. However, given that these markers are influenced by various systemic factors, their association with OA may not provide conclusive evidence. While our predictive model demonstrated strong accuracy, the findings should be interpreted with caution, recognizing the complexity of these biomarkers’ roles. Further longitudinal research is necessary to validate these associations and to explore their utility in personalized OA treatment strategies, ensuring that they are not confounded by other systemic conditions.
Quantifying mineral levels along with biomarkers could serve as a valuable adjunct for diagnosing OA, enhancing its diagnostic accuracy and potentially informing personalized treatment strategies.
References
- 1.Angelini F, Widera P, Mobasheri A, Blair J, Struglics A, Uebelhoer M, et al. Osteoarthritis endotype discovery via clustering of biochemical marker data. Ann Rheum Dis 2022;81:666-75. [Google Scholar]
- 2.Saberi Hosnijeh F, Siebuhr AS, Uitterlinden AG, Oei EH, Hofman A, Karsdal MA, et al. Association between biomarkers of tissue inflammation and progression of osteoarthritis: Evidence from the Rotterdam study cohort. Arthritis Res Ther 2016;18:81. [Google Scholar]
- 3.Antony B, Singh A. Imaging and biochemical markers for osteoarthritis. Diagnostics (Basel) 2021;11:1205. [Google Scholar]
- 4.Li G, Cheng T, Yu X. The impact of trace elements on osteoarthritis. Front Med (Lausanne) 2021;8:771297. [Google Scholar]
- 5.Khann V. Is calcium supplementation required in osteoarthritis patients? Adv Res Gastroenterol Hepatol 2016;1:555575. [Google Scholar]
- 6.Frestedt JL, Kuskowski MA, Zenk JL. A natural seaweed derived mineral supplement (Aquamin F) for knee osteoarthritis: A randomised, placebo controlled pilot study. Nutr J 2009;8:7. [Google Scholar]
- 7.Nguyen C, Bazin D, Daudon M, Chatron-Colliet A, Hannouche D, Bianchi A, et al. Revisiting spatial distribution and biochemical composition of calcium-containing crystals in human osteoarthritic articular cartilage. Arthritis Res Ther 2013;15:R103. [Google Scholar]
- 8.Liew JW, Jarraya M, Guermazi A, Lynch J, Wang N, Rabasa G, et al. Relation of intra-articular mineralization to knee pain in knee osteoarthritis: A longitudinal analysis in the MOST study. Arthritis Rheumatol 2023;75:2161-8. [Google Scholar]
- 9.Xia F, Li Q, Luo X, Wu J. Identification for heavy metals exposure on osteoarthritis among aging people and Machine learning for prediction: A study based on NHANES 2011-2020. Front Public Health 2022;10:906774. [Google Scholar]
- 10.Rodríguez J, Mandalunis PM. A review of metal exposure and its effects on bone health. J Toxicol 2018;2018:e4854152. [Google Scholar]
- 11.Guan T, Wu Z, Xu C, Su G. The association of trace elements with arthritis in US adults: NHANES 2013-2016. J Trace Elem Med Biol 2023;76:127122. [Google Scholar]
- 12.Oyakhire F, Abiodun EM, Ajileye SA, Egho EV, Osaro E, Benjamin II, et al. Evaluation of micronutrients and vitamins in patients diagnosed with osteoarthritis. Med Sci Discov 2022;9:153-63. [Google Scholar]
- 13.Yazar M, Sarban S, Kocyigit A, Isikan UE. Synovial fluid and plasma selenium, copper, zinc, and iron concentrations in patients with rheumatoid arthritis and osteoarthritis. Biol Trace Elem Res 2005;106:123-32. [Google Scholar]