Low-grade myofibroblastic sarcomas (LGMS) are rare tumors, found commonly in the peripheral skeleton. Diagnosis of these tumors is a challenge due to specific immunohistochemical markers related to them and their rarity. Vigilance relating to all diagnostic modalities is a pre-requisite to correct treatment protocols, with targeted pre-operative planning to embolize the feeding vessels of the tumor as well. This is a rare case of LGMS presenting in the axial skeleton
Dr. Shataayu Gugale, Department of Orthopaedics, Mahatma Gandhi Medical College and Hospital, Jaipur, Rajasthan, India. E-mail: shataayu07@gmail.com
Introduction: Low-grade myofibroblastic sarcoma (LGMS) is an atypical and extremely infrequent type of tumor, primary mass being usually present in subcutaneous and soft tissue. Bony involvement is very rare. It has a very high chance of recurrence locally due to its aggressive biological behavior, metastasis in other parts of body is rarely seen. On X-ray, it is visualized as an osteolytic and destructive mass. On magnetic resonance imaging (MRI) this tumor shows heterogenous high signal intensity in T2 images and hypo- to iso-intensity in T1 images. Histologically, these tumors present as diffuse, infiltrative growth pattern traversing between muscle fibers, and cellular atypia with singular mitotic figures. These are obligatory criteria for diagnosis. Due to diagnostic difficulty including histopathological limiting factors, the treatment protocol for LGMS s is still challenging.
Case Report: A 21-year-old female with pain in right pelvic and hip region since past 6 months presented with a diffuse swelling over her right hip, along with tenderness over greater trochanter, groin and buttock region. On basis of MRI findings, core needle biopsy was performed for diagnosis and the sample was suggestive of a spindle cell lesion composed of cells arranged in haphazard and vaguely fascicular pattern. To confirm the diagnosis immunohistochemistry marker study was done, revealing features suggestive of LGMS (smooth muscle actin: Positive, B-cell lymphoma 2: Positive, Desmin: Negative, H-caldesmon: Negative). Histomorphological and immune-histo-chemical features showed a final diagnosis of LGMS of right-sided acetabulum. Positron emission tomography -scan was done to rule out any distant metastasis, and it did not show any definitive evidence of abnormal hyper-metabolism elsewhere in the body. Pre-operative computed tomography (CT) angiography was done and plan was made to embolize the feeding vessels preoperatively using the findings of CT angiography.
Conclusion: Presentation of LGMS with bone involvement or bone lesions is very rare. Clinicoradiological diagnosis be misinterpreted as a benign lesion which can lead to insufficient resection and local recurrence of the tumor. For treatment in such cases, most studies emphasize on excision of tumor with a wide surgical margin, but regarding the safety distance for better adequacy, relevant data is still inconsistent. In our case, we have reported a case of a 21-year-old female for gross total resection and reconstruction of an acetabular LGMS with extension into iliac wing. To the best of our knowledge, it is the first case of this tumor involving the acetabulum.
Keywords: Low-grade myofibroblastic sarcoma, acetabulum surgeries, axial skeleton tumours.
Low-grade myofibroblastic sarcoma (LGMS) is an atypical and extremely infrequent type of tumor which usually involves head and neck region of body [1]. It was first described by Mentzel et al. in 1988 [2]. In 2002 the World Health Organization included this tumor in International Classification of Diseases for Oncology, [1] and this classification was maintained until up to 2020 [1]. Primary tumor mass is usually present in subcutaneous and soft tissue, and bony involvement is very rare [3]. It has a very high chance of recurrence locally due to its aggressive biological behavior but metastasis in other parts of body is rarely seen [4]. For initial investigations, X-rays and magnetic resonance imaging (MRI) (with contrast) are typically used. On X-ray, if bone involvement is present it visualize as osteolytic and destructive mass, while sometimes calcification and ossification of soft tissue can be seen on X-ray. On MRI, this tumor shows heterogenous high signal intensity in T2 images and hypo to iso-intensity in T1 images as compared to muscles [5]. Histologically, LGMS are presented as diffuse infiltrative growth pattern traversing between muscle fibers, cellular atypia with singular mitotic figures are obligatory criteria for diagnosis [6]. Use of electron microscopy is very limited in LGMS because of high cost and unavailability. Mentzel and Schneider-Stock described that LGMS presents positively with smooth muscle actin (SMA), B-cell lymphoma (BCL2) positive and can be focally positive for cluster of differentiation 34 and calponin [7]. Hence with above mentioned diagnostic difficulty including histopathological limiting factor, treatment protocol for LGMS is still challenging. Primary LGMS seems to be extremely rare and around 16 cases being reported affecting the bone in the literature previously [8]. For better illustration of this, we present a case of LGMS of acetabulum in a 21-year-old female where wide resection of tumor as well as preserving future functional ability of limb was the mainstay of treatment. To the best of our knowledge, this is the first case worldwide of LGMS which includes acetabular bony involvement.
History and course in the hospital
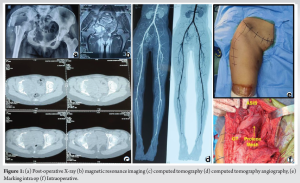
A 21-year-old female was presented to our hospital with pain in the right hip and pelvic region from past 6 months. Pain was dull aching in nature, and because of continued progression of pain she had difficulty in walking since the past 3 months. On physical examination, a diffuse swelling was present over her right hip, along with tenderness over the greater trochanter, groin and buttock region. On examination, the right hip range of movement was severely limited compared to the normal left hip. No significant personal and family history was present. Other systemic examinations were within normal limits. Her pain score was 8/10 on Visual Analogue Scale (VAS) scale. On radiological investigations, X-ray of pelvis with both hips showing osteolytic involvement of the right acetabulum area (Fig. 1a). MRI showed a soft-tissue mass centered in the right acetabulum involving acetabular margin of the right hip joint. This mass appeared hypointense in T1-weighted images with central area of hyper-intensity suggesting blood products, and hyper-intense on T2 and short tau inversion recovery images, giving an appearance similar to aneurysmal bone cysts (Fig. 1b). Computed tomography (CT) was done to see the bony involvement of the area and it showed expansile lobulated lytic mass lesion centered in the right acetabulum involving acetabular margins of hip joint (Fig. 1c).
On basis of radiological finding, core needle biopsy was performed for diagnosis and the sample was suggestive of spindle cell lesions composed of cells arranged in haphazard and vaguely fascicular pattern and they had minimal pleomorphism, no mitotic figures and no necrosis. To confirm the diagnosis, immunohistochemistry (IHC) marker study was done and it revealed features suggestive of LGMS (SMA: Positive, BCL2: Positive, Desmin: Negative, H-caldesmon: Negative).
On evaluation of imaging, histomorphological and immune-histo-chemical features a final diagnosis of LGMS of acetabulum right side was confirmed. Positron emission tomography-scan was done to rule out any distant metastasis but it did not show any definitive evidence of abnormal hyper-metabolism elsewhere in the body. On considering all above findings, decision was taken in form of wide excision of the tumor.
These types of pelvic tumor usually bleed too much during surgery. To overcome this problem, a pre-operative CT angiography was done (Fig. 1d) and plan was made to embolizing the feeding vessels preoperatively using the findings of CT angiography. The patient was finally taken for surgery after all pre-operative tests with anesthetic fitness and pre-embolization of feeding vessels, and appropriate consents regarding the procedure and prognosis were taken from the patient and her attendants before procedures.
Operation
The patient was operated by a multi-disciplinary team of surgeons from departments of orthopedics and surgical oncology. The ilioinguinal approach along with an extended iliofemoral approach was combined in the form of a T-shaped incision to approach the tumor (Fig. 1e). The tumor was identified and resected all around while taking margins of 3–3.5 cm meanwhile preserving the neurovascular structures, mainly the femoral vessels and the sciatic nerve. The cut started from the pubic bone near the pubic tubercle. Next, anterior super iliac spine was osteotomized and iliac bone was cut from the anterior margin till the sciatic notch. Posterior cut was taken just distal to the ischial spine. Pelvic resection Type 1, 2, and 3 (Enneking classification) were combined [9].
Next, patient position was changed to floppy lateral from supine and greater trochanteric was osteotomized to gain further access to the tumor. Capsule of hip joint was incised all around and the ligamentum teres was cut to free the pelvic segment from the femur. Now margins from each bone along with marrow and soft tissues were sent for histopathological examination with frozen sections. When all sections came back negative, the surgical field was washed with normal saline and hemostasis was achieved.
Now, a 30 × 30 cm prolene mesh was taken and molded according to the defect just created, and fixed to the bone as well as the soft tissue with the help of absorbable sutures so as to repair the defect, while keeping the neurovascular structures in mind and being careful not to injure them (Fig. 1f). The osteotomies along with the muscular attachments were repaired and the wound closed.
Post-operative course
Our patient had a long post-operative in hospital course wherein she responded well to physiotherapy. She was able to stand with the help of a walker on post-operative day 3 and was given a hip abduction brace for added support to her limb. Adjuvant chemotherapy or radiotherapy was not used for this patient. The patient underwent MRI follow-up 6 months after surgery and MRI showed no local recurrence, able to ambulate with assistance of a walker and is doing well.
Modified Harris Hip score was improved from 40 pre-operatively to 78 postoperatively, in 6 month post-operative. While VAS for pain improved from 8 preoperatively to 1 postoperatively 6 months.
The patient had provided informed consent for publication of the case, and the study protocol was approved by Medical Ethics Committee of our institution (Table 1).
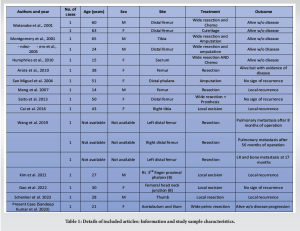
Myofibroblasts are spindle cells of mesenchymal origin which share the features of smooth muscles as well as fibroblastic cells. They are known to participate in many physiological processes such as wound healing but are also cells of origin in some benign tumors of soft tissues [6]. Mentzel et al. first reported in 1998 a malignant lesion of myofibroblastic sarcoma originating from this cell type [2]. LGMS is a very uncommon and underestimated tumor that can present in the axial skeleton. However, reported cases of the tumor are also very low due to difficulty in diagnosis. In the literature also limited number of case series and case reports are present. In the present study, we have systematically collected all data related to LGMS involving bone lesions from the literature, it is most commonly present in head and neck regions, but literature has suggested that it can be found in every region of the body [8, 10-23]. Study of Schenker et al. identified a total of 60 cases in extremities, out of them 16 have bony involvement (Table 1) [8]. In the literature, no distinct age preference was found however study from Chan JY et al. suggested that older patients have poor survival rate compared to younger patients [24]. For diagnosis of this rare tumor, both MRI and histopathological evaluation is essential. MRI can easily rule out a benign lesion from LGMS and can help prevent an unnecessary aggressive procedure in the form of surgery [25]. For a definitive diagnosis, the combined approach involving the histopathological and IHC analysis is must [26, 27]. For treatment in LGMS cases, most studies emphasize on excision of tumor with a wide surgical margin, but the safety distance for better adequacy and relevant data are still inconsistent. However, Kim et al. suggested a routine resection margin of 3 cm is adequate [23]. Chang et al. suggested that the local relapse rate was 22% in case of LGMS involving the extremities. [3]. Some treating doctors used radiotherapy, chemotherapy or adjuvant treatment with surgery, but a study conducted by Holevich J et al. indicated that in case of LGMS patients, no supportive evidence was present for pre or post-operative chemotherapy or adjuvant therapy if we achieved negative surgical margins during the surgery [28, 29]. In the present case, pelvic resection Type 1, 2, and 3 Enneking and Dunham [9] were combined and the defect was repaired with a prolene mesh. Other prosthetic implants like saddle prosthesis could not be used and pelvic resection was chosen as an appropriate treatment, after taking into consideration the age of the patient and her financial constraints.
Presentation of low-grade myofibroblastic sarcoma (LGMS) with bone involvement or bone lesions is very rare. Clinico-radiological experience can be misdiagnosed as a benign lesion which can lead to insufficient resection and local recurrence. Proper histopathological and immunohistochemical studies are necessary for diagnosis. The primary goal of surgery is to achieve a tumor-free margin and in the case of extremities, function-preserving surgeries should be desirable. In our case, we report a case of a 21-year-old female for gross total resection and reconstruction of an acetabular LGMS with extension into iliac wing. To the best of our knowledge, it is the first case of LGMS involving the acetabulum with regards to published literature. The goal of our treatment was to prolong progression-free survival and to improve the quality of life and functional ability of the patient.
LGMSs are rare tumors; presentations with musculoskeletal involvements are seldom encountered. They can be misdiagnosed, leading to insufficient resection and local recurrence. Histopathological vigilance and correct immune-histochemical studies are a must for correct diagnosis. Treatment modalities should be centered around tumor excision with wide surgical margin, and timely follow-ups are necessitated to check for tumor recurrence.
References
- 1.WHO Classification of Tumours Editorial Board. WHO Classification of Tumours: Soft Tissue and Bone Tumours. Lyon, France: International Agency for Research on Cancer, IARD Press; 2020. [Google Scholar]
- 2.Mentzel T, Dry S, Katenkamp D, Fletcher CD. Low-grade myofibroblastic sarcoma: Analysis of 18 cases in the spectrum of myofibroblastic tumors. Am J Surg Pathol 1998;22:1228-38. [Google Scholar]
- 3.Chang SE, Choi JH, Sung KJ, Moon KC, Koh JK, Lee TJ, et al. A case of cutaneous low-grade myofibroblastic sarcoma. J Dermatol 2001;28:383-7. [Google Scholar]
- 4.Oylumlu M, Yildiz A, Ercan S, Oylumlu M, Davutoglu V. Cardiac metastasis of a low-grade myofibroblastic sarcoma. Echocardiography 2014;31:E1-4. [Google Scholar]
- 5.Wang L, Li LX, Chen DQ, Yang L, Li SK, Cheng C. Low-grade myofibroblastic sarcoma: Clinical and imaging findings. BMC Med Imaging 2019;19:36. [Google Scholar]
- 6.Vasudev KS, Harris M. A sarcoma of myofibroblasts: An ultrastructural study. Arch Pathol Lab Med 1978;102, 185-8. [Google Scholar]
- 7.Mentzel T, Schneider-Stock R. Niedrigmalignes myofibroblastisches sarkom. In: Pathologie: Kopf-Hals-Region, Weichgewebstumoren, Haut. 3rd ed. Berlin/Heidelberg, Germany: Springer; 2009. [Google Scholar]
- 8.Schenker A, Gutjahr E, Lehner B, Mechtersheimer G, Wardelmann E, Klotz R, et al. A systematic review and illustrative case presentation of low-grade myofibroblastic sarcoma (LGMS) of the extremities. J Clin Med 2023;12:7027. [Google Scholar]
- 9.Enneking WF, Dunham WK. Resection and reconstruction for primary neoplasms involving the innominate bone. J Bone Joint Surg Am 1978;60:731-46. [Google Scholar]
- 10.Gao G, Liu Y, Ao Y, Wang J, Xu Y. Low-grade myofibroblastic sarcoma of the proximal femur: A case report and literature review. Medicine (Baltimore) 2022;101:e31715. [Google Scholar]
- 11.Watanabe K, Ogura G, Tajino T, Hoshi N, Suzuki T. Myofibrosarcoma of the bone: A clinicopathologic study. Am J Surg Pathol 2001;25:1501-7. [Google Scholar]
- 12.Diaz-Cascajo C, Borghi S, Weyers W, Metze D. Fibroblastic/myofibroblastic sarcoma of the skin: A report of five cases. J Cutan Pathol 2003;30:128-34. [Google Scholar]
- 13.Roth TM, Fratkin J, Woodring TC, McGehee RP. Low-grade myofibroblastic sarcoma of the vulva. Gynecol Oncol 2004;92:361-64. [Google Scholar]
- 14.Humphries WE 3rd, Satyan KB, Relyea K, Kim ES, Adesina AM, Chintagumpala M, et al. Low-grade myofibroblastic sarcoma of the sacrum. J Neurosurg Pediatr 2010;6:286-90. [Google Scholar]
- 15.Morgan PB, Chundru S, Hatch SS, Hawkins HK, Adegboyega PA, Eltorky MA. Uncommon malignancies: Case 1. Low-grade myofibroblastic sarcoma of the breast. J Clin Oncol 2005;23:6249-51. [Google Scholar]
- 16.Tavora F, Miettinen M, Fanburg-Smith J, Franks TJ, Burke A. Pulmonary artery sarcoma: A histologic and follow-up study with emphasis on a subset of low-grade myofibroblastic sarcomas with a good long-term follow-up. Am J Surg Pathol 2008;32:1751-61. [Google Scholar]
- 17.Meng GZ, Zhang HY, Zhang Z, Wei B, Bu H. Myofibroblastic sarcoma vs nodular fasciitis: A comparative study of chromosomal imbalances. Am J Clin Pathol 2009;131:701-9. [Google Scholar]
- 18.Montgomery E, Goldblum JR, Fisher C. Myofibrosarcoma: A clinicopathologic study. Am J Surg Pathol 2001;25:219-28. [Google Scholar]
- 19.San Miguel P, Fernandez G, Ortiz-Rey JA, Larrauri P. Low-grade myofibroblastic sarcoma of the distal phalanx. J Hand Surg Am 2004;29:1160-3. [Google Scholar]
- 20.Arora R, Gupta R, Sharma A, Dinda AK. A rare case of low-grade myofibroblastic sarcoma of the femur in a 38-year-old woman: A case report. J Med Case Rep 2010;4:121. [Google Scholar]
- 21.Saito T, Mitomi H, Kurisaki A, Torigoe T, Takagi T, Suehara Y, et al. Low-grade myofibroblastic sarcoma of the distal femur. Int J Surg Case Rep 2013;4:195-9. [Google Scholar]
- 22.Cai ZG, Pan CC, Yu DH, Feng ZZ, Ma L, Zhao Y, et al. Myofibroblastic sarcomas: A clinicopathologic analysis of 15 cases and review of literature. Int J Clin Exp Pathol 2016;9:1568-77. [Google Scholar]
- 23.Kim JH, Choi W, Cho HS, Lee KS, Park JK, Kim BK. Surgical treatment and long-term outcomes of low-grade myofibroblastic sarcoma: A single-center case series of 15 patients. World J Surg Oncol 2021;19:339. [Google Scholar]
- 24.Chan JY, Gooi Z, Wong EW, Ng SK, Tong MC, Vlantis AC. Low-grade myofibroblastic sarcoma: A population-based study. Laryngoscope 2017;127:116-21. [Google Scholar]
- 25.Scalas G, Parmeggiani A, Martella C, Tuzzato G, Bianchi G, Facchini G, et al. Magnetic resonance imaging of soft tissue sarcoma: Features related to prognosis. Eur J Orthop Surg Traumatol 2021;31:1567-75. [Google Scholar]
- 26.Yonezawa H, Yamamoto N, Hayashi K, Takeuchi A, Miwa S, Igarashi K, et al. Low-grade myofibroblastic sarcoma of the levator scapulae muscle: A case report and literature review. BMC Musculoskelet Disord 2020;l21:836. [Google Scholar]
- 27.Nagata Y, Matsuno T, Hamada N, Shimose S, Arihiro K, Ochi M. Low-grade myofibroblastic sarcoma of the palm. Scand J Plast Reconstr Surg Hand Surg 2008;42:164-7. [Google Scholar]
- 28.Holevich, J. Our technique of pedicle skin flaps and its use in the surgery of the hand and fingers. Acta Chir Plast 1960;2:271-84. [Google Scholar]
- 29.Holevich J, Paneva-Holevich E. Bipedicled island flap. Acta Chir Plast 1971;13:106-16. [Google Scholar]