Medical management of Fibrous Dysplasia is limited and surgical management in children should be customized according to the site, extent, and presentation of the disease. Recurrence of lesions is common and difficult to predict, it is advisable to follow all children till skeletal maturity for recurrence
Dr. Ankur Agarwal, Department of Pediatric Orthopaedics, Postgraduate Institute of Child Health, Sector 30, Noida - 201303, Uttar Pradesh, India. E-mail: drankur@gmail.com
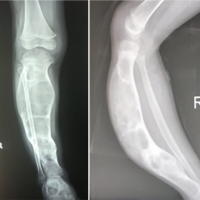
Introduction: Fibrous dysplasia (FD) accounts for 5–7% of all benign bone tumors. It presents in two main forms – monostotic, which is more common affecting a single bone and presenting usually in 3rd decade of life; polyostotic, affecting several bones, is less common, and is seen mainly in the 1st decade of life. These usually present as bone pain or pathological fracture. It may also be part of McCune-Albright syndrome. Since the femur is an important weight-bearing bone in the human body, most cases of FD affecting the femur present as pathological fracture more early than other sites. The mainstay of management includes treatment of pathological fracture and prevention of bony deformity until skeletal maturity. The aim of this study was to analyze a series of cases of FD of femur affecting the pediatric population.
Materials and Methods: A retrospective study was conducted at two tertiary-level centers of urban India, wherein all case records of FD under follow-up between January 2015 and December 2023 were analyzed. In all, there were 13 cases. Definitive management was decided on a case-by-case basis. We grouped the cases into 2 categories based on recurrence. Observations: Out of 13 cases, 11 were males while 2 patients were females. The mean age at presentation was 10.5 years (range 4–12 years). Two cases were of polyostotic FD, while 11 cases were of monostotic FD. In 11 cases, the intertrochanteric region of the femur was affected, while in 2 cases the affection extended into the proximal shaft of the femur as well. The mean follow-up period was 48 months (Range 15–84 months). In category one (no recurrence) there were 10 patients, while in category two (recurrence of FD) there were 3 patients. The recurrence rate in our series was 23%.
Results: Although FD is also seen in the adult population, its implications are more pronounced in a growing skeleton. The series of cases where surgical intervention has been done with a long follow-up in the pediatric population are limited. In literature, clinical classification of FD, anatomical location in the proximal femur, variation in neck-shaft angle and osteocalcin levels have been found to be significant in predicting causes of fractures and their recurrences.
Conclusion: Internal fixation is preferable to prevent deformities where there are high chances of a pathological fracture as is evident by the natural history of FD. Long-term follow-up is important as there are chances of recurrence in childhood until puberty.
Keywords: Fibrous dysplasia, proximal femur, shepherd crook deformity, pamidronate, monostotic, polyostotic, Mccune-Albright syndrome.
Fibrous dysplasia (FD) accounts for 5–7% of all benign bone tumors [1]. The affected bone gets replaced by a fibrous stroma with cellular components consisting of mutated fibroblasts and osteoblasts of varying functionality. This results in abnormal shaped trabeculae of woven bone. It is presented in two main forms [2]. Monostotic form, which is more common (75% of cases), affects single bone and usually presents in 3rd decade of life. Polyostotic form affecting several bones is less common (25% of presenting cases) [3]. It is seen mainly in the 1st decade of life. It mainly affects cranio-facial bones, ribs, femur, and sometimes tibia. The clinical presentation is usually with bone pain or a pathological fracture. The entity is genetically linked to GNAS1 gene mutation; however, it is non-inherited [4]. It may also be part of McCune Albright syndrome (MAS) which is a clinical triad of FD, café-au-lait skin spots, and precocious puberty. It becomes dormant as the child moves into adulthood [5]. Plain radiographic picture is typical of thinned out bone with irregular radiolucent or areas with ground glass appearance. Magnetic resonance imaging (MRI) is diagnostic as it identifies areas and extent of fibrous affection within the bone [6]. The management is directed at pain control, improvement of bone density, and management of a pathological fracture [7,8]. The risk of recurrence is 18% approximately while the risk of malignant transformation is 1–4% [9]. Since the femur is an important weight-bearing bone of the human body, most cases of FD affecting the femur present as pathological fractures more early than other sites of affection. The mainstay of management includes management of pathological fracture and prevention of bony deformity until skeletal maturity. The aim of this study was to analyze a series of cases of FD of the femur affecting the pediatric population – presentation, management, and medium-term follow-up.
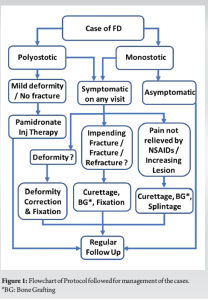
A retrospective study was conducted at two tertiary level centers of urban India, wherein all cases records of FD of bone presenting and under follow-up between January 2015 and December 2023 were analyzed. In all, there were 16 cases, 3 adult cases were excluded from the present study. In all cases, basic workup in the form of plain radiography, screening X-rays of other parts, advanced imaging, hematological workup, and pediatric consultation was carried out. Advanced imaging studies mostly included non-contrast MRI. In all cases, the diagnosis was confirmed by biopsy and histopathology. Definitive management was decided on a case-by-case basis. Our management protocol is summarized in Fig. 1. We grouped the cases into 2 categories based on recurrence. In category one, we had patients who did not have a recurrence of lesion or refracture till the latest follow-up. In category two, there were patients who had a recurrence after initial treatment which required intervention. Since the existing literature does not specifically define recurrence in FD, for our series we defined it as regression of radiological signs of progressive healing with or without fracture or a deformity.
Observations
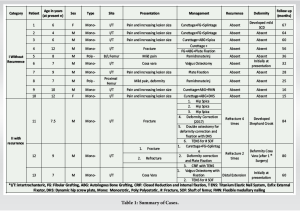
Table 1 summarizes the observations in our series of cases of FD. Out of 13 cases, 11 were males while 2 patients were females. The mean age at presentation was 10.5 years (range 4–12 years). Two cases were of polyostotic FD, while 11 cases were of monostotic FD. In 11 cases, the intertrochanteric region of the femur was affected, while in 2 cases the affection extended into the proximal shaft of the femur as well. The mean follow-up period was 48 months (Range 15–84 months).
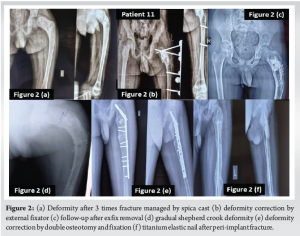
In category one, there were 10 patients. Two patients (patient number 5 and 8) presented with mild pain and deformity. They had a polyostotic type of FD. Both patients were treated with an injection of pamidronate (1 mg/mL). Before initiation of therapy, it was ensured that the patient was normo-calcemic, with normal 25-OH Vitamin D levels (30–100 ng/mL), normal creatinine clearance (>35 mL/min), and hypophosphatemia, if any, has been pharmacologically corrected. Pamidronate was used in a dosage of 1 mg/kg was given for three consecutive days. The cycle was repeated after 8–12 weeks interval. It was decided that the therapy was to be discontinued if there was no response after two cycles. However, both our patients showed a clinical response and the lesions did not progress till the latest follow-up. Eight patients presented with either fracture or impending fracture due to a lesion in the proximal femur. All these patients were treated with bone grafting and fixation of fracture. In all these patients there was no pathological fracture or recurrence of lesion after primary surgery till the latest follow-up. In category two, there were three patients (patients 11, 12, and 13). All these patients had a history of multiple fractures and deformities. Patient 11 (Fig. 2) was a 7 and 1/2 year-old child when he developed a fracture of his femur in the year 2015. He was treated with hip spica for 3 months at a nearby hospital for the fracture. However, as he started mobilization there was a refracture of the femur. He had three episodes of fracture in 1 and 1/2 years. Every time he was treated with hip spica. He came to us in the year 2017 with a deformity of proximal femur. He was operated on with deformity correction in the year 2017. He again had a pathological fracture in 2020, at this time fracture was mismanaged due to the COVID-19 pandemic and he developed severe shepherd crook deformity. In 2021 patient was treated with deformity correction and fixation in 2021, unfortunately, he developed a foot drop due to stretching of common peroneal nerve during surgery. However, the nerve recovered gradually but the patient developed a pathological fracture for the 5th time, for which he was treated with titanium elastic nail (TENS). Till the latest follow-up, the patient is mobilizing without support.
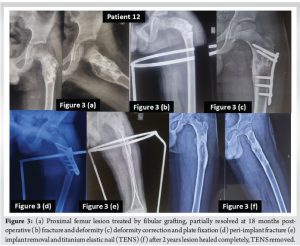
Patient no 12 (Fig. 3) was a 9-year-old child who was operated on in the years 2015 and 2016 with bone grafting for a lesion in the proximal femur. However, the lesion progressed with time, and he developed a deformity of the proximal femur along with a pathological fracture at the age of 12 years in the year 2018. He was operated on with deformity correction and fixation with a valgus plate. Eight months after the third surgery patient developed a peri-implant fracture, which was further operated with plate removal and TENS. TENS were removed after fracture union in the year 2020. The child is still under follow-up and lesions have healed completely, and the child is completely asymptomatic now.
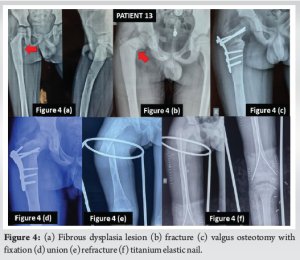
Patient 13 (Fig. 4) was a 7-year-old boy who had a history of trauma to his thigh for which he was treated with plaster at a nearby hospital in year 2018. After 1 year of trauma, the child developed a limp which was increasing progressively. The patient came to us in 2020 with a limp and a fracture of neck of the femur. He was operated with a valgus osteotomy at that time. The fracture united and the limp improved with time. The implant was removed 18 months after fracture union. In 2022 he again developed a fracture shaft of the femur which was treated with TENS. Until the latest follow-up, fracture has been united completely and the child is asymptomatic.
Although FD is also seen in the adult population, its implications are more pronounced in a growing skeleton. Several series have been presented in the literature. However, the series of cases where surgical intervention has been done with a long follow-up in the pediatric population are limited. FD in child can present with either monostotic or polyostotic variance. Skeletal survey can easily differentiate between monostotic and polyostotic variant. Since misdiagnosis of FD is quite common to the tune of 17%, we confirmed all of the radiological and MRI suspected cases by histopathology. Chondroma, chondromatosis, intra-osseous angioma, osteo FD, ossifying fibroma, and non-ossifying fibroma may mimic in imaging to FD. Histopathology and sometimes molecular genetics are required for accurate diagnosis, especially in monostotic FD [10]. In polyostotic FD, a careful endocrinological workup is important to decipher the associated MAS. Most of the cases in our series presented with a deformity or pathological fracture or had a high risk of pathological fracture requiring operative intervention and fixation. There is a general agreement on surgical management of such affections [11,12,13]. Tathe et al. in their series of 6 patients of monostotic FD affecting the proximal femur used an extramedullary implant for fixation [14]. Zhang et al. classified FD of the femur into 5 radiological types based on bone strength and proximal femoral deformity. They advocated prophylactic surgery and internal fixation to prevent deformity in Type 2 femurs [15]. Although intramedullary nailing has been recommended by many studies as the preferred treatment in such cases, the choice of fixation in our series depended on the location of the lesion and fracture [16]. Ippolito et al. managed 21 patients (29 femurs and 14 tibias) of polyostotic FD with intramedullary nailing. Their indication for surgical intervention was a painful limp, deformity, or fracture [17]. We did not undertake internal fixation for painful limp. In our series, 8 out of 13 patients (62%) had pain as presenting complaints. Curettage, with autogenous bone grafting, was carried out with splintage. All healed without any recurrence till the past follow-up, except one patient who presented with a fracture, and fixation was done subsequently. Thus, internal fixation was not primary management for those cases of FD who presented with pain alone as a symptom. Ippolito et al. also found in their series that intravenous bisphosphonate injection was helpful only in relieving pain associated with FD. It did not strengthen the bone and the deformity progressed leading to fracture in some cases despite bisphosphonate therapy [17]. Similar findings were there in a study by Leet et al. [18]. This contrasts with what Lala et al. observed in their series of nine cases [19]. In our series, we had 2 patients of polyostotic FD, who were treated with intravenous Pamidronate bisphosphonate therapy. In both patients, pain improved significantly and there was no pathological fracture till the past follow-up, though the mild progression of the deformity was there in one patient (patient number 8). Thus, bisphosphonates do have a role in FD, especially in polyostotic form, but the extent of their role is still debatable, as no study has shown them to increase local bone density or preventing complications. Pamidronate and zolendronate have been shown to be most efficacious [12]. In a series by Ippolito et al., repeat intra medullary nailing was carried out in more than two-thirds of the cases with additional surgical procedures in fourteen cases for deformity correction and equalizing limb lengths. They also converted intramedullary nailing to interlocking nailing in patients with poor follow-up compliance and those near to physeal closure [17]. In our series, additional procedures were carried out only for recurrences in 3 out of 13 patients (23%). Ippolito et al., did not recommend curettage with bone grafting of FD lesion as they anticipated resorption of bone graft and replacement by FD tissue [17]. In our series, we carried out autogenous bone grafting in 3 out of 13 patients. None of these showed any recurrence. We used fibular strut graft in 4 out of 13 patients. Only one of these showed recurrence in the form of a fracture. DiCaprio and Enneking have also reported successful use of cortical strut grafts in monostotic FD [1]. Most studies also suggest increased blood loss during FD surgical procedures especially those conducted for curettage, and deformity correction; more so in the femur [20,21]. In our series also, we anticipated moderate blood loss during surgery, especially in the femur where a tourniquet could not be applied. Blood loss was reduced by the use of hypotensive anesthesia. Blood transfusion was not required in any case. A relatively smaller number of cases in our study could be a statistical limitation; however, this number of cases of monostotic FD is comparable to the series of Ippolito et al., with a reasonably long follow-up. Our study has focused only on cases of FD affecting the proximal femur area and that too in the pediatric population. The difference with other studies is that we tailor-made treatment on a case-to-case basis because of the socioeconomic concerns of a developing country, rather than a single uniform approach followed by Ippolito et al. This tailor-made approach could; however, act as a confounding factor in assessing the final outcomes of the study. The effect of this confounding factor was reduced by the fact that decision-making was done through deliberation by a single unit protocol which was well defined before the start of the study as depicted in Fig. 1. Another limitation of our study is that we did not ascertain the cause of recurrence in our patients. Clinical classification of FD, anatomical location in proximal femur, variation in neck-shaft angle and osteocalcin levels have been found to be significant in predicting causes of fractures and their recurrences in certain subset of patients with FD [22]. Recurrences may also be linked to GNAS gene mutation [4]. Additional studies including genetic testing can be carried out in our second category patients, i.e., with recurrences to ascertain causes of repeat fractures. Almost all literature points to having a long-term follow-up of the patients, especially in childhood, as there are high chances of recurrence, especially till skeletal maturity. The recurrence rate in our series was twenty-three percent. This is in comparison to other studies done with long-term follow-up [17]. The mean follow-up of our study was 48 months at the time of the past follow-up. This could be a limitation of our study as not all cases were followed up till puberty at the close of the study, which is important in cases of FD.
FD in children is a challenging clinical entity and a high recurrence rate is common. Medical treatment is limited and mainly focused on pain relief and to improve bone quality. Internal fixation is preferable to prevent deformities where there are high chances of a pathological fracture as is evident by the natural history. Fixation becomes mandatory if a pathological fracture has already occurred. Long-term follow-up is important as there are chances of recurrence until puberty. Further studies to ascertain the causes of recurrence may provide useful insight into disease management.
FD in children is difficult to manage as a high recurrence rate is a common phenomenon. Medical treatment of FD is limited and mainly focused on pain relief and to improve bone quality. There are no uniform guidelines for surgical management of varied presentation of FD in children and it must be customized according to site and extent of the disease. All patients should be followed till skeletal maturity for recurrence.
References
- 1.DiCaprio MR, Enneking WF. Fibrous dysplasia. Pathophysiology, evaluation, and treatment. J Bone Joint Surg Am 2005;87:1848-64. [Google Scholar | PubMed]
- 2.Feller L, Wood NH, Khammissa RA, Lemmer J, Raubenheimer EJ. The nature of fibrous dysplasia. Head Face Med 2009;5:22. [Google Scholar | PubMed]
- 3.Riddle ND, Bui MM. Fibrous dysplasia. Arch Pathol Lab Med 2013;137:134-8. [Google Scholar | PubMed]
- 4.Tang J, Zhao HY, Zheng L, Zhang HZ, Jiang ZM. Abnormal expression of c-myc, p53, p16 protein and GNAS1 gene mutation in fibrous dysplasia. Zhonghua Bing Li Xue Za Zhi 2009;38:292-7. [Google Scholar | PubMed]
- 5.Dahlin DC. Bone Tumors: General Aspects and Data on 6,221 Cases. 3rd ed. Springfield, IL: Thomas; 1978. p.445. [Google Scholar | PubMed]
- 6.Shah ZK, Peh WC, Koh WL, Shek TW. Magnetic resonance imaging appearances of fibrous dysplasia. Br J Radiol 2005;78:1104-15. [Google Scholar | PubMed]
- 7.Stanton RP. Surgery for fibrous dysplasia. J Bone Miner Res 2006;21 Suppl 2:P105-9. [Google Scholar | PubMed]
- 8.Chapurlat RD, Gensburger D, Jimenez-Andrade JM, Ghilardi JR, Kelly M, Mantyh P. Pathophysiology and medical treatment of pain in fibrous dysplasia of bone. Orphanet J Rare Dis 2012;7 Suppl 1:S3. [Google Scholar | PubMed]
- 9.MacDonald-Jankowski D. Fibrous dysplasia: A systematic review. Dentomaxillofac Radiol 2009;38:196-215. [Google Scholar | PubMed]
- 10.Ippolito E, Bray EW, Corsi A, De Maio F, Exner UG, Robey PG, et al. Natural history and treatment of fibrous dysplasia of bone: A multicenter clinicopathologic study promoted by the European pediatric orthopaedic society. J Pediatr Orthop B 2003;12:155-77. [Google Scholar | PubMed]
- 11.Andrisano A, Soncini G, Calderoni PP, Stilli S. Critical review of infantile fibrous dysplasia: Surgical treatment. J Pediatr Orthop 1991;11:478-81. [Google Scholar | PubMed]
- 12.Javaid MK, Boyce A, Appelman-Dijkstra N, Ong J, Defabianis P, Offiah A, et al. Best practice management guidelines for fibrous dysplasia/McCune-Albright syndrome: A consensus statement from the FD/MAS international consortium. Orphanet J Rare Dis 2019;14:139. [Google Scholar | PubMed]
- 13.Benedetti Valentini M, Ippolito E, Catellani F, Farsetti P. Internal fixation after fracture or osteotomy of the femur in young children with polyostotic fibrous dysplasia. J Pediatr Orthop B 2015;24:291-5. [Google Scholar | PubMed]
- 14.Tathe PV, Banik S, Mandal S. Surgical treatment modalities in pediatric monostotic fibrous dysplasia of proximal femur-a case series. J Orthop Case Rep 2022;12:13-8. [Google Scholar | PubMed]
- 15.Zhang X, Chen C, Duan H, Tu C. Radiographic classification and treatment of fibrous dysplasia of the proximal femur: 227 femurs with a mean follow-up of 6 years. J Orthop Surg Res 2015;10:171. [Google Scholar | PubMed]
- 16.Majoor BC, Leithner A, Van de Sande MA, Appelman-Dijkstra NM, Hamdy NA, Dijkstra PD. Individualized approach to the surgical management of fibrous dysplasia of the proximal femur. Orphanet J Rare Dis 2018;13:72. [Google Scholar | PubMed]
- 17.Ippolito E, Farsetti P, Caterini R, Micciulli E, Gorgolini G, Ruzzini L. Intramedullary nailing for lower limb polyostotic fibrous dysplasia in children: A long-term follow-up study. J Pediatr Orthop 2022;42:e492-500. [Google Scholar | PubMed]
- 18.Leet AI, Collins MT. Current approach to fibrous dysplasia of bone and McCune-Albright syndrome. J Child Orthop 2007;1:3-17. [Google Scholar | PubMed]
- 19.Lala R, Matarazzo P, Bertelloni S, Buzi F, Rigon F, De Sanctis C. Pamidronate treatment of bone fibrous dysplasia in nine children with McCune-Albright syndrome. Acta Paediatr 2000;89:188-93. [Google Scholar | PubMed]
- 20.Bianco P, Gehron Robey P, Wientroub S. Fibrous dysplasia of bone. In: Glorieux HJ, editor. Pediatric Bone Biology and Disease. New York: Academic Press;2003. 509-539. [Google Scholar | PubMed]
- 21.Enneking WF, Gearen PF. Fibrous dysplasia of the femoral neck. J Bone Joint Surg Am 1986;68A:1415-22. [Google Scholar | PubMed]
- 22.Liu W, Xu M, Yu X. Risk factors for fracture in patients with fibrous dysplasia of the proximal femur. J Int Med Res 2022;50:(12). 3000605221142395. [Google Scholar | PubMed]