This case highlights the effectiveness of staged surgical interventions and targeted antifungal therapy, particularly fluconazole, in achieving favorable outcomes in fungal prosthetic joint infection
Dr. Sharafuddeen Mammu, Department of Orthopaedic Surgery, Fathima Hospital, Kozhikode, Kerala, India. E-mail: Sharafuddeen786@gmail.com
Introduction: Fungal infections following total knee replacement surgeries are rare but present significant challenges in management due to their delayed diagnosis and resistance to standard antimicrobial therapies.
Case Report: This case report describes the management of a delayed prosthetic joint infection in a 65-year-old female, diagnosed as a fungal infection 4 years after total knee replacement. Initially presented with persistent knee pain, swelling, and restricted range of motion despite standard antimicrobial therapy, further investigation revealed fungal elements in synovial fluid analysis, confirming the diagnosis. The patient underwent a staged procedure involving debridement, application of antifungal and antibiotic cement spacer, followed by delayed revision surgery. A 12-week course of antifungal fluconazole therapy was administered postoperatively. Subsequent 1-year follow-ups revealed symptomatic improvement and the absence of infection recurrence.
Conclusion: This case highlights the efficacy of staged surgical interventions and targeted antifungal therapy in achieving favorable outcomes for fungal prosthetic joint infections. It underscores the importance of long-term follow-up for monitoring and surveillance in such cases.
Keywords: Prosthetic joint infection, amphotericin B, fluconazole, inflammatory markers, Candida.
Total knee arthroplasty (TKA) represents one of the most commonly performed orthopedic procedures worldwide. Osteoarthritis, which is the main cause of TKA, limits joint movement and affects millions of patients [1]. Infections following total knee replacement surgeries represent a dreaded consequence, with detrimental effects on functional outcomes and heightened morbidity. Instances of fungal infection after joint replacement are not common. Unlike bacterial infections, which are more frequently encountered, fungal prosthetic joint infections are characterized by delayed diagnosis, insidious progression, and limited response to conventional antimicrobial therapies. Due to their rarity and the complex interplay of host factors and fungal virulence, these infections often pose diagnostic dilemmas for clinicians, leading to delays in appropriate treatment initiation. The consequences of untreated fungal prosthetic joint infections can be severe, potentially resulting in implant failure, chronic pain, functional impairment, and systemic complications. Furthermore, the limited availability of effective antifungal agents and the necessity for prolonged courses of therapy add further layers of complexity to the management of these cases [2,3]. Among the diverse spectrum of fungal pathogens, various Candida species have been implicated in prosthetic joint infections, contributing to the complexity of diagnosis and treatment. Candida species, including Candida albicans, Candida glabrata, Candida parapsilosis, and Candida tropicalis, exhibit varying degrees of virulence and resistance to antifungal agents, necessitating tailored therapeutic approaches. Understanding the epidemiology, pathogenesis, and clinical manifestations of fungal infections caused by different Candida species is crucial for accurate diagnosis and effective management. Recent studies highlight the emergence of Candida auris as an increasingly recognized pathogen in health-care settings, further complicating the therapeutic landscape of fungal infections [4,5]. Moreover, advancements in molecular diagnostic tools, such as next-generation sequencing, have revolutionized the detection of fungal pathogens in culture-negative cases, enhancing diagnostic accuracy and clinical outcomes [6,7]. This review aims to provide an overview of fungal infections following knee replacement surgeries, with a focus on the role of Candida species as causative agents, their antimicrobial susceptibility profiles, and emerging treatment strategies.
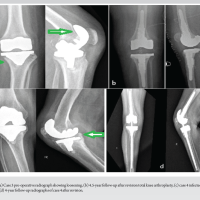
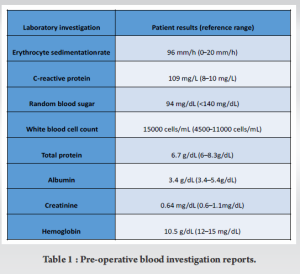
A 65-year-old female who had a symptom-free period of 4 years following a right total knee replacement (posterior stabilized knee system by Maxx freedom), developed a gradual onset of right knee pain accompanied by swelling, which was managed conservatively by a local general physician. She presented to our outpatient department with severe right knee pain and limping, clinical examination revealed effusion over the knee and a local rise in temperature. Blood investigations demonstrated elevated total white blood cell count and inflammatory markers, indicating a potential infectious etiology (Table 1). A radiograph of the knee joint showed loose femoral and tibial components and osteolysis (Fig. 1). Knee joint aspiration conducted from the operation theater under aseptic precautions revealed serosanguinous effusion with particles. Fluid samples were sent for bacterial and fungal culture, alongside counts and synovial inflammatory markers, to ascertain the underlying cause. Despite negative bacterial culture results, para-aminosalicylic acid staining and fungal culture identified the presence of a colony of C. parapsilosis. As a result, a two-staged revision procedure was planned. This approach was chosen due to the severity of the infection and the need for thorough eradication of the pathogen.
First stage: Thorough debridement and placement of antibiotic and antifungal-coated cement spacer
Thorough debridement
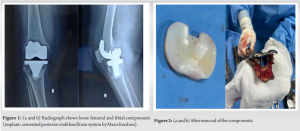
The first stage involved meticulous debridement of the infected tissue surrounding the knee joint. This process aimed to remove any necrotic or infected tissue, as well as biofilm formations on the prosthetic components. Following debridement, the prosthetic components of the knee joint were carefully removed (Fig. 2).
Placement of antibiotic and antifungal-coated cement spacer
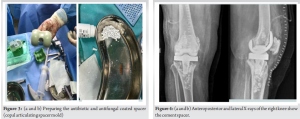
After prosthesis removal, antibiotic and antifungal-coated cement spacers were placed in the joint space. The spacer contained a mixture of amphotericin B 150 mg, vancomycin 2 g, and tobramycin 320 mg (Fig. 3). This spacer served multiple purposes: It provided local delivery of antimicrobial agents to the affected area, maintained joint space integrity, and facilitated eradication of the infection (Fig. 4).
Confirmation of diagnosis
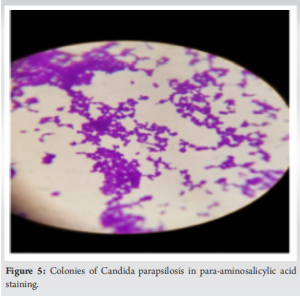
Intraoperative culture repetition was performed to confirm the diagnosis of C. parapsilosis infection (Fig. 5) and ensure appropriate management.
Oral antifungal therapy
Before the second stage, the patient was initiated on oral fluconazole 150 mg for a 3-month duration. This systemic antifungal therapy aims to eradicate any residual fungal infection and prevent recurrence. Intravenous amphotericin B was avoided due to its serious systemic side effects.
Revision surgery as second stage
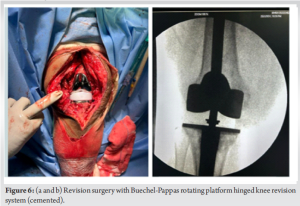
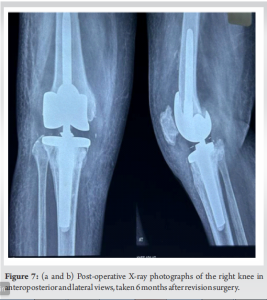
Once inflammatory markers normalized and the infection was deemed under control, after 3 months revision surgery was undertaken. This procedure involved the removal of the antibiotic and antifungal-coated cement spacer and placement of a new prosthetic hinge knee joint with Buechel-Pappas rotating platform hinged revision knee system (Fig. 6). Thromboprophylaxis measures were implemented to reduce the risk of deep vein thrombosis. Post-revision surgery, the patient underwent mobilization using a walking frame under the guidance of physical therapists. Clinical assessment and repeat X-rays were taken at 1, 3, and 6 months (Fig. 7). At the 1-year follow-up, the patient was able to perform her routine daily activities with a pain-free gait and knee movement ranging from 0 to 100° of flexion. Inflammatory markers, including erythrocyte sedimentation rate and C-reactive protein, were found to be normal. There was no knee joint effusion, and the X-ray showed a well-fitted implant.
Joint infection after knee replacement is debilitating to the patient. Most etiological organisms causing infection are bacterial species, mainly Staphylococcus aureus and coagulase-negative staphylococci. Fungal prosthetic joint infections are extremely rare. Despite the use of infection control strategies, the risk of infection following joint replacement is 1–2%, of which fungal infection is estimated to cause 1% of all prosthesis infections [8]. Fungal prosthetic joint infection is known to occur in patients who are immune compromised, have an underlying systemic illness, or have prolonged use of antibiotics [9,10]. Our patient had no comorbidities/risk factors. The common organism causing mycotic infection is C. albicans. In our case, the causative organism was found to be C. parapsilosis. Wu and Hsu reported a patient with pre-operative cutaneous candidiasis who developed a candidal joint infection [11]. In the presented case, a primary source of candidal infection remains elusive. Given the prolonged interval of over 4 years since the primary TKA and the recent onset of symptoms within the past few weeks, the likelihood of infection being introduced during the initial surgery appears exceedingly low. The pathogenicity of Candida is considered a result of its ability to secrete hydrolytic enzymes and form a biofilm on the prosthesis surface that protects it from systemic antifungals [12]. The diagnosis of prosthetic joint infection relies on a thorough history and clinical examination. Elevated total counts and raised inflammatory markers suggest the presence of infection. Obtaining multiple aspirate specimens aids in better sampling for accurate diagnosis. In cases where bacterial culture is negative despite clinical suspicion, a high index of suspicion for fungal infection should be maintained. New molecular diagnostic tools, such as next-generation sequencing, have shown promise in identifying fungal pathogens in culture-negative infections, improving diagnostic accuracy [13]. When there is a strong clinical suspicion, along with confirmatory culture-based and radiographic evidence, the patient should undergo revision surgery, ideally opting for a two-stage revision arthroplasty. Radiological evaluation, in most cases, shows loosening of the implant, osteolysis, or local bone destruction [14]. Debridement with prosthesis retention or resection arthroplasty has shown high failure and revision rates [15]. Due to the limited literature available on this condition, there is no established standard management guideline to adhere to. However, most studies recommend two-stage revision surgery with the use of antifungal-loaded cement spacers and antifungal therapy. Following the initial operation, most patients undergo at least 6 weeks of systemic antifungal therapy. There is no specific drug of choice for the condition or specific guidelines to follow as the reported cases are less. The drug of choice for fungal joint infection is controversial. Amphotericin B is considered the gold standard, but side effects, especially nephrotoxicity may limit its use, particularly for the long term. Fluconazole is an alternative with fewer side effects [16]. Fluconazole has been shown to present fewer adverse effects and is suitable for long-term use in patients. There have been documented cases of successful treatment using fluconazole as the sole antifungal medication. Its high bioavailability, prolonged half-life, minimal occurrence of severe side effects, and substantial concentration in joint fluid render fluconazole a favorable option. Recent trials have explored the efficacy of echinocandins, such as caspofungin, in managing fungal prosthetic joint infections, showing encouraging results. In our case, there was no notable slimy layer at the interface of the bone cement. Nevertheless, biofilms do develop in fungal infections just as they do in bacterial infections.
It is evident that fungal periprosthetic joint infection (PJI), while rare, poses a significant challenge and is notably more intricate to address compared to bacterial PJI. The management of fungal prosthetic joint infection with staged revision prosthesis and antifungals remains a challenging but crucial aspect of orthopedic care. Despite the limited literature available, a two-stage revision surgery with an antifungal-loaded cement spacer coupled with systemic antifungal therapy, notably using fluconazole, appears to be a commonly recommended approach. This underscores the importance of a multidisciplinary approach involving infectious disease specialists, orthopedic surgeons, and microbiologists to tailor treatment strategies to individual patient needs. Continued research efforts are warranted to further elucidate optimal management strategies and improve outcomes for patients with fungal prosthetic joint infections.
There is importance of early recognition and aggressive management of fungal prosthetic joint infections, as it is rare. Staged revision prosthesis combined with appropriate antifungal therapy gives the best results. There is a pressing need for the establishment of robust protocols for both diagnosis and management to effectively tackle this serious complication in orthopedic practice.
References
- 1.Cobo F, Rodríguez-Granger J, López EM, Jiménez G, Sampedro A, Aliaga-Martínez L, et al. Candida-induced prosthetic joint infection: A literature review including 72 cases and a case report. Infect Dis (Lond) 2017;49:81-94. [Google Scholar | PubMed]
- 2.Dutronc H, Dauchy FA, Cazanave C, Rougie C, Lafarie-Castet S, Couprie B, et al. Candida prosthetic infections: Case series and literature review. Scand J Infect Dis 2010;42:890-5. [Google Scholar | PubMed]
- 3.Hwang BH, Yoon JY, Nam CH, Jung KA, Lee SC, Han CD, et al. Fungal peri-prosthetic joint infection after primary total knee replacement. J Bone Joint Surg Br 2012;94:656-9. [Google Scholar | PubMed]
- 4.Jeffery-Smith A, Taori SK, Schelenz S, Jeffery K, Johnson EM, Borman A, et al. Candida auris: A review of the literature. Clin Microbiol Rev 2018;31:e00029-17. [Google Scholar | PubMed]
- 5.Osei Sekyere J. Candida auris: A systematic review and meta-analysis of current updates on an emerging multidrug-resistant pathogen. MicrobiologyOpen 2018;7:e00578. [Google Scholar | PubMed]
- 6.Gonzalez Moreno M, Coronado Rivera AM, Senneville E, Bernard L, Veltman ES, Ferry T. Echinocandins for fungal prosthetic joint infections: A systematic review. J Fungi (Basel) 2021;7:589. [Google Scholar | PubMed]
- 7.Kohli A, Khanna T, Kapadia B, Brown PJ, Parvizi J. Two-stage revision for fungal periprosthetic joint infection: A systematic review. J Arthroplasty 2019;34:1019-27. [Google Scholar | PubMed]
- 8.Brooks DH, Pupparo F. Successful salvage of a primary total knee arthroplasty infected with Candida parapsilosis. J Arthroplasty 1998;13:707-12. [Google Scholar | PubMed]
- 9.Wu MH, Hsu KY. Candidal arthritis in revision knee arthroplasty successfully treated with sequential parenteral-oral fluconazole and amphotericin B-loaded cement spacer. Knee Surg Sports Traumatol Arthrosc 2011;19:273-6. [Google Scholar | PubMed]
- 10.Jakobs O, Schoof B, Klatte TO, Schmidl S, Fensky F, Guenther D, et al. Fungal periprosthetic joint infection in total knee arthroplasty: A systematic review. Orthop Rev (Pavia) 2015;7:5623. [Google Scholar | PubMed]
- 11.Azzam K, Parvizi J, Jungkind D, Hanssen A, Fehring T, Springer B, et al. Microbiological, clinical, and surgical features of fungal prosthetic joint infections: A multi-institutional experience. J Bone Joint Surg Am 2009;91:142-9. [Google Scholar | PubMed]
- 12.Reddy KJ, Shah JD, Kale RV, Reddy TJ. Fungal prosthetic joint infection after total knee arthroplasty. Indian J Orthop 2013;47:526-9. [Google Scholar | PubMed]
- 13.Binkley CE, Chen AF. Next-generation sequencing for diagnosis of periprosthetic joint infection. J Bone Jt Infect 2019;4:131-7. [Google Scholar | PubMed]
- 14.Chandra J, Mukherjee PK, Ghannoum MA. Fungal biofilms and their role in human disease. J Fungi (Basel) 2021;7:453. [Google Scholar | PubMed]
- 15.Dutronc H, Rougie C, Lafarie-Castet S, Couprie B, Fabre T, Dupon M. Candida-induced prosthetic joint infection: Review and management perspectives. Clin Infect Dis 2022;74:489-95. [Google Scholar | PubMed]
- 16.Gonzalez Moreno M, Ferry T, Coronado Rivera AM, Bernard L. Systematic review of the management of fungal periprosthetic joint infections. J Bone Jt Infect 2021;6:1-9. [Google Scholar | PubMed]